Methylammonium chloride
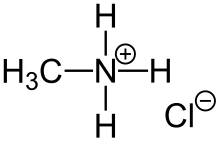
Methylammonium chloride in an organic halide with a formula of CH3NH3Cl. It is an ammonium salt composed of methylamine and hydrogen chloride. One potential application for the methylammonium halides is in the production of perovskite solar cells.[3][4] The methyl group and other hydrogen atoms are bonded covalently to the nitrogen, with the chloride bonded ionically.
 | |
| Names | |
|---|---|
| IUPAC name
Methylazanium chloride | |
| Systematic IUPAC name
Methanaminium chloride | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.906 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3NH3Cl | |
| Molar mass | 67.51804 g/mol |
| Appearance | White crystals [1] |
| Hazards[2] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
irritant |
| GHS labelling: | |
 | |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- "Methylammonium chloride". Greatcell Solar Materials. Retrieved 10 April 2021.
- "GESTIS-Stoffdatenbank". gestis.dguv.de. Retrieved 11 March 2021.
- Li, Hangqian. (2016). "A modified sequential deposition method for fabrication of perovskite solar cells". Solar Energy. 126: 243–251. Bibcode:2016SoEn..126..243L. doi:10.1016/j.solener.2015.12.045.
- Zhao, X. (2021). "Methylammonium Chloride reduces the bandgap width and trap densities for efficient perovskite photodetectors". Journal of Materials Science. 56: 9242–9253. doi:10.1007/s10853-021-05840-2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.