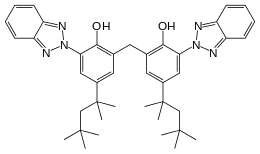
Bisoctrizole
Bisoctrizole (INN[1]/USAN,[2] marketed by BASF as Tinosorb M, by DSM Nutritional Products as Parsol Max , by Everlight Chemical as Eversorb M, and by MPI as Milestab 360, INCI methylene bis-benzotriazolyl tetramethylbutylphenol) is a phenolic benzotriazole that is added to sunscreens to absorb UV rays.[3] It is a broad-spectrum ultraviolet radiation absorber, absorbing UVB as well as UVA rays.[3] It also reflects and scatters UV.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-Methylenebis[6-(2H-1,2,3-benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol] | |
| Other names
UV-360 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.100.550 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C41H50N6O2 | |
| Molar mass | 658.88 g/mol |
| Melting point | 195.7 °C (384.3 °F; 468.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bisoctrizole is what is termed is a hybrid UV absorber, which has been described as an organic UV filter produced in microfine organic particles (< 200 nm),[4][5] like microfine zinc oxide and titanium dioxide. Where other organic UV absorbers dissolved in either the oily or aqueous phases, bisoctrizole dissolves poorly in both.
Hence, bisoctrizole is formulated in sunscreen preparations as a 50% suspension, the absorber added to the water phase, and mineral micropigments usually added to the oil phase. The bisoctrizole particles are stabilized by the surfactant decyl glucoside. The compound shows very little photodegradation, and has a stabilizing effect on other UV absorbers, octyl methoxycinnamate (octinoxate) in particular.
In primary research reports, bisoctrizole has been reported to minimally penetrate skin,[6] and has been described as lacking estrogenic effects in vitro.[7]
As of this date, bisoctrizole had not been approved by the U.S. Food and Drug Administration (FDA), but is approved in the EU and other parts of the world.[8][9][10]
References
- WHO Staff (2005). "Recommended International Nonproprietary Names: List 54, International Nonproprietary Names for Pharmaceutical Substances (INN) [Entry 'bisoctrizolum']". WHO Drug Information. 19 (3). Archived from the original on October 19, 2009. Retrieved July 5, 2022.
- National Library of Medicine Staff (July 5, 2022). "Bisoctrizole". Chem.NLM.NIH.gov. Retrieved July 5, 2022.
- Latha, MS; Martis, Jacintha; Shobha, V; Shinde, Rutuja Sham; Bangera, Sudhakar; Krishnankutty, Binny; Bellary, Shantala; Varughese, Sunoj; Rao, Prabhakar; Naveen Kumar, B.R. (January 2013). "Sunscreening Agents A Review". Journal of Clinical and Aesthetic Dermatology. 6 (1): 16–26. PMC 3543289. PMID 23320122.
- Ciba Staff (July 5, 2022). "TINOSORB® M, Broad-spectrum UV Filter for the Water Phase". BASF.com. Retrieved July 5, 2022.
- Herzog, B.; Mongiat, S.; Deshayes, C.; Neuhaus, M.; Sommer, K.; Mantler, A. (2002). "In vivo and in vitro assessment of UVA protection by sunscreen formulations containing either butyl methoxy dibenzoyl methane, methylene bis-benzotriazolyl tetramethylbutylphenol, or microfine ZnO". International Journal of Cosmetic Science. 24 (3): 170–85. doi:10.1046/j.1467-2494.2002.00137.x. PMID 18498509. S2CID 37553401.
- Mavon A, Miquel C, Lejeune O, Payre B, Moretto P (2007). "In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen". Skin Pharmacol Physiol. 20 (1): 10–20. doi:10.1159/000096167. PMID 17035717. S2CID 22041398.
- Ashby J, Tinwell H, Plautz J, Twomey K, Lefevre PA (December 2001). "Lack of binding to isolated estrogen or androgen receptors, and inactivity in the immature rat uterotrophic assay, of the ultraviolet sunscreen filters Tinosorb M-active and Tinosorb S". Regul Toxicol Pharmacol. 34 (3): 287–91. doi:10.1006/rtph.2001.1511. PMID 11754532.
- Kapes, Beth (July 1, 2005). "Docs Rally for Better Sun Protection". ModernMedicine.com. Archived from the original on October 9, 2007. Retrieved July 5, 2022.
- "Eur-Lex.Europa.eu PDF" (PDF). Archived from the original (PDF) on 2008-08-14. Retrieved 2007-08-19.
- Australian Regulatory Guidelines for OTC Medicines, Chapter 10.
External links
- Ciba® TINOSORB® M – Microfine UV absorber with Triple Action
- http://www.dermatologytimes.com/dermatologytimes/article/articleDetail.jsp?id=159652
- NEW-WAVE SUNSCREENS – Active ingredient makers are frustrated by the long list of sunscreens and UV-A testing protocols that are still awaiting FDA decisions, C&EN Cover Story
- https://www.fda.gov/ohrms/dockets/dockets/05n0446/05n-0446-bkg0001-03-Tab-01-vol2.pdf