Mucosal immunology
Mucosal immunology is the study of immune system responses that occur at mucosal membranes of the intestines, the urogenital tract, and the respiratory system.[1] The mucous membranes are in constant contact with microorganisms, food, and inhaled antigens.[2] In healthy states, the mucosal immune system protects the organism against infectious pathogens and maintains a tolerance towards non-harmful commensal microbes and benign environmental substances.[1] Disruption of this balance between tolerance and deprivation of pathogens can lead to pathological conditions such as food allergies, irritable bowel syndrome, susceptibility to infections, and more.[2]
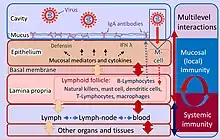
The mucosal immune system consists of a cellular component, humoral immunity, and defense mechanisms that prevent the invasion of microorganisms and harmful foreign substances into the body. These defense mechanisms can be divided into physical barriers (epithelial lining, mucus, cilia function, intestinal peristalsis, etc.) and chemical factors (pH, antimicrobial peptides, etc.).[3]
Function
The mucosal immune system provides three main functions:
- First line of defense from harmful antigenic structures and infection.[3]
- Prevents systemic immune responses to commensal bacteria and food antigens.[2]
- Regulates appropriate immune responses to pathogens.[2]
Physical barrier
Mucosal barrier integrity physically stops pathogens from entering the body.[4] Barrier function is determined by factors such as age, genetics, types of mucins present on the mucosa, interactions between immune cells, nerves and neuropeptides, and co-infection. Barrier integrity depends on the immunosuppressive mechanisms implemented on the mucosa.[3] The mucosal barrier is formed due to the tight junctions between the epithelial cells of the mucosa and the presence of the mucus on the cell surface.[4] The mucins that form mucus offer protection from components on the mucosa by static shielding and limit the immunogenicity of intestinal antigens by inducing an anti-inflammatory state in dendritic cells (DC).[5]
Active immunity
Because the mucosa surfaces are in constant contact with external antigens and microbiota many immune cells are required. For example, approximately 3/4 of all lymphocytes are found in the mucous membranes.[3] These immune cells reside in secondary lymphoid tissue, largely distributed through the mucosal surfaces.[3]
The mucosa-associated lymphoid tissue (MALT), provides the organism with an important first line of defense. Along with the spleen and lymph nodes, the tonsils and MALT are considered to be secondary lymphoid tissue.[7]
The MALT's cellular component is composed mostly of dendritic cells, macrophages, innate lymphoid cells, mucosal-associated invariant T cells, intraepithelial T cells, regulatory T cells (Treg), and IgA secreting plasma cells.[1][3][8]
Intraepithelial T cells, usually CD8+, reside between mucosal epithelial cells. These cells do not need primary activation like classic T cells. Instead, upon recognition of antigen, these cells initiate their effector functions, resulting in faster removal of pathogens.[8] Tregs are abundant on the mucous membranes and play an important role in maintaining tolerance through various functions, especially through the production of anti-inflammatory cytokines.[9] Mucosal resident antigen-presenting cells (APCs) in healthy people show a tolerogenic phenotype.[10] These APCs do not express TLR2 or TLR4 on their surfaces. In addition, only negligible levels of the LPS receptor CD14 are normally present on these cells.[10] Mucosal dendritic cells determine the type of subsequent immune responses by the production of certain types of cytokines and the type of molecules involved in the co-stimulation.[3] For example production of IL-6 and IL-23 induce Th17 response,[4] IL-12, IL-18 and INF-γ induce Th1 response,[3][4] IL-4 induces Th2 response,[4] and IL-10, TGF-β and retinoic acid induce tolerance.[11] Innate lymphoid cells are abundant in the mucosa where via rapid cytokine production in response to tissue-derived signals, they act as regulators of immunity, inflammation, and barrier homeostasis.[12]
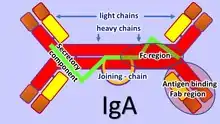
The adaptive mucosal immune system is involved in maintaining mucosal homeostasis through a mechanism of immune exclusion mediated by secretory antibodies (mostly IgA) that inhibit the penetration of invasive pathogens into the body's tissues and prevent the penetration of potentially dangerous exogenous proteins.[13] Another mechanism of adaptive mucosal immunity is the implementation of immunosuppressive mechanisms mediated mainly by Tregs to prevent local and peripheral hypersensitivity to harmless antigens, i.e. oral tolerance.[11]
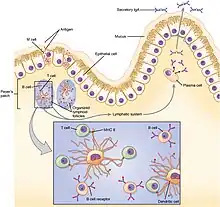
Basic immune response in the gut
In the gut, lymphoid tissue is dispersed in gut-associated lymphoid tissue (GALT). A large number of immune system cells in the intestines are found in dome-like structures called Peyer’s patches and in small mucosal lymphoid aggregates called cryptopatches.[14] Above the Peyer’s patches is a layer of epithelial cells, which together with the mucus form a barrier against microbial invasion into the underlying tissue. Antigen sampling is a key function of Peyer’s patches. Above the Peyer’s patches is a much thinner mucus layer that helps the antigen sampling.[14] Specialized phagocytic cells, called M cells, which are found in the epithelial layer of the Peyer’s patches, can transport antigenic material across the intestinal barrier through the process of transcytosis.[15] The material transported in this way from the intestinal lumen can then be presented by the antigen-presenting cells present in Peyer’s patches.[14][15] In addition, dendritic cells in Peyer’s patches can extend their dendrites through M cell-specific transcellular pores and they can also capture translocated IgA immune complexes.[16] Dendritic cells then present the antigen to naïve T cells in the local mesenteric lymph nodes.[17]
If mucosal barrier homeostasis has not been violated and invasive pathogens are not present, dendritic cells induce tolerance in the gut due to induction of Tregs by secretion of TGF-β and retinoic acid.[17] These Tregs further travel to the lamina propria of villi through lymphatic vessels. There, Tregs produce IL-10 and IL-35, which affects other immune cells in the lamina propria toward a tolerogenic state.[17]
However, damging the homeostasis of the intestinal barrier leads to inflammation. The epithelium in direct contact with bacteria is activated and begins to produce danger-associated molecular patterns (DAMPs).[17] Alarm molecules released from epithelial cells activate immune cells.[17][18] Dendritic cells and macrophages are activated in this environment and produce key pro-inflammatory cytokines such as IL-6, IL-12, and IL-23 which activate more immune cells and direct them towards a pro-inflammatory state.[18] The activated effector cells then produce TNF, IFNγ, and IL-17.[18] Neutrophils are attracted to the affected area and begin to perform their effector functions.[1] After the ongoing infection has been removed, the inflammatory response must be stopped to restore homeostasis.[17] The damaged tissue is healed and everything returns to its natural state of tolerance.[17]
Neonatal
At birth, neonates' mucosal immune systems are relatively undeveloped and need intestinal flora colonies to promote development.[7] Microbiota composition stabilizes around the age of 3.[2] In the neonatal period and in early childhood interaction of host immunity with the microbiome is critical. During this interaction various immunity arms are educated. They contribute to homeostasis and determine the future immune system settings, i.e. its susceptibility to infections and inflammatory diseases.[2][3] For example, the B cell line in the intestinal mucosa is regulated by extracellular signals from commensal microbes that affect the intestinal immunoglobulin repertoire.[19] Diversity of microbiota in early childhood protects the body from the induction of mucosal IgE, which is associated with allergy development.[20]
Mucosal vaccines
Because of its front-line status within the immune system, the mucosal immune system is being investigated for use in vaccines for various afflictions, including COVID-19,[21][22][23][24][25] HIV,[26] allergies, poliovirus, influenza A and B, rotavirus, vibrio cholerae and many others.[27][28]
See also
References
- "Mucosal immunology - Latest research and news". Nature Portfolio. Springer Nature Limited. Retrieved 2016-11-08.
- Zheng D, Liwinski T, Elinav E (June 2020). "Interaction between microbiota and immunity in health and disease". Cell Research. 30 (6): 492–506. doi:10.1038/s41422-020-0332-7. PMC 7264227. PMID 32433595.
- Brandtzaeg P (2009). Brandtzaeg P, Isolauri E, Prescott SL (eds.). "'ABC' of mucosal immunology". Nestle Nutrition Workshop Series. Paediatric Programme. Nestlé Nutrition Institute Workshop Series. 64: 23–38, discussion 38–43, 251–7. doi:10.1159/000235781. ISBN 978-3-8055-9167-6. PMID 19710513.
- Okumura R, Takeda K (December 2018). "Maintenance of intestinal homeostasis by mucosal barriers". Inflammation and Regeneration. 38 (1): 5. doi:10.1186/s41232-018-0063-z. PMC 5879757. PMID 29619131.
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. (October 2013). "Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals". Science. 342 (6157): 447–453. Bibcode:2013Sci...342..447S. doi:10.1126/science.1237910. PMC 4005805. PMID 24072822.
-
 This article incorporates text available under the CC BY 4.0 license. Betts, J Gordon; Desaix, Peter; Johnson, Eddie; Johnson, Jody E; Korol, Oksana; Kruse, Dean; Poe, Brandon; Wise, James; Womble, Mark D; Young, Kelly A (September 13, 2023). Anatomy & Physiology. Houston: OpenStax CNX. 21.5 The immune response against pathogens. ISBN 978-1-947172-04-3.
This article incorporates text available under the CC BY 4.0 license. Betts, J Gordon; Desaix, Peter; Johnson, Eddie; Johnson, Jody E; Korol, Oksana; Kruse, Dean; Poe, Brandon; Wise, James; Womble, Mark D; Young, Kelly A (September 13, 2023). Anatomy & Physiology. Houston: OpenStax CNX. 21.5 The immune response against pathogens. ISBN 978-1-947172-04-3. - Torow N, Marsland BJ, Hornef MW, Gollwitzer ES (January 2017). "Neonatal mucosal immunology". Mucosal Immunology. 10 (1): 5–17. doi:10.1038/mi.2016.81. PMID 27649929. S2CID 3556125.
- Olivares-Villagómez D, Van Kaer L (April 2018). "Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier". Trends in Immunology. 39 (4): 264–275. doi:10.1016/j.it.2017.11.003. PMC 8056148. PMID 29221933.
- Richert-Spuhler LE, Lund JM (2015). "The Immune Fulcrum: Regulatory T Cells Tip the Balance Between Pro- and Anti-inflammatory Outcomes upon Infection". Progress in Molecular Biology and Translational Science. Elsevier. 136: 217–243. doi:10.1016/bs.pmbts.2015.07.015. ISBN 978-0-12-803415-6. PMC 4769439. PMID 26615099.
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. (March 2011). "Succession of microbial consortia in the developing infant gut microbiome". Proceedings of the National Academy of Sciences of the United States of America. 108 (Supplement_1): 4578–4585. Bibcode:2011PNAS..108.4578K. doi:10.1073/pnas.1000081107. PMC 3063592. PMID 20668239.
- Traxinger BR, Richert-Spuhler LE, Lund JM (November 2021). "Mucosal tissue regulatory T cells are integral in balancing immunity and tolerance at portals of antigen entry". Mucosal Immunology. 15 (3): 398–407. doi:10.1038/s41385-021-00471-x. PMC 8628059. PMID 34845322.
- Sonnenberg GF, Hepworth MR (October 2019). "Functional interactions between innate lymphoid cells and adaptive immunity". Nature Reviews. Immunology. 19 (10): 599–613. doi:10.1038/s41577-019-0194-8. PMC 6982279. PMID 31350531.
- Chen K, Magri G, Grasset EK, Cerutti A (July 2020). "Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA". Nature Reviews. Immunology. 20 (7): 427–441. doi:10.1038/s41577-019-0261-1. PMC 10262260. PMID 32015473. S2CID 211017339.
- Mörbe UM, Jørgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, Agace WW (July 2021). "Human gut-associated lymphoid tissues (GALT); diversity, structure, and function". Mucosal Immunology. 14 (4): 793–802. doi:10.1038/s41385-021-00389-4. PMID 33753873. S2CID 232322692.
- Dillon A, Lo DD (2019-07-02). "M Cells: Intelligent Engineering of Mucosal Immune Surveillance". Frontiers in Immunology. 10: 1499. doi:10.3389/fimmu.2019.01499. PMC 6614372. PMID 31312204.
- Stagg AJ (2018-12-06). "Intestinal Dendritic Cells in Health and Gut Inflammation". Frontiers in Immunology. 9: 2883. doi:10.3389/fimmu.2018.02883. PMC 6291504. PMID 30574151.
- Tordesillas L, Berin MC (October 2018). "Mechanisms of Oral Tolerance". Clinical Reviews in Allergy & Immunology. 55 (2): 107–117. doi:10.1007/s12016-018-8680-5. PMC 6110983. PMID 29488131.
- Chistiakov DA, Bobryshev YV, Kozarov E, Sobenin IA, Orekhov AN (2015-01-13). "Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance". Frontiers in Microbiology. 5: 781. doi:10.3389/fmicb.2014.00781. PMC 4292724. PMID 25628617.
- Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, et al. (September 2013). "Microbial colonization influences early B-lineage development in the gut lamina propria". Nature. 501 (7465): 112–115. Bibcode:2013Natur.501..112W. doi:10.1038/nature12496. PMC 3807868. PMID 23965619.
- Integrative HMP (iHMP) Research Network Consortium; Proctor, Lita M.; Creasy, Heather H.; Fettweis, Jennifer M.; Lloyd-Price, Jason; Mahurkar, Anup; Zhou, Wenyu; Buck, Gregory A.; Snyder, Michael P.; Strauss, Jerome F.; Weinstock, George M.; White, Owen; Huttenhower, Curtis (May 2019). "The Integrative Human Microbiome Project". Nature. 569 (7758): 641–648. Bibcode:2019Natur.569..641I. doi:10.1038/s41586-019-1238-8. PMC 6784865. PMID 31142853.
- Mandavilli, Apoorva (2 Feb 2022). "The Covid Vaccine We Need Now May Not Be a Shot". The New York Times. Retrieved 18 November 2022.
- Mueller, Benjamin (18 Nov 2022). "The End of Vaccines at 'Warp Speed'". The New York Times. Retrieved 18 November 2022.
- Tang, J; Zeng, C; Cox, TM; Li, C; Son, YM; Cheon, IS; Wu, Y; Behl, S; Taylor, JJ; Chakaraborty, R; Johnson, AJ; Shiavo, DN; Utz, JP; Reisenauer, JS; Midthun, DE; Mullon, JJ; Edell, ES; Alameh, MG; Borish, L; Teague, WG; Kaplan, MH; Weissman, D; Kern, R; Hu, H; Vassallo, R; Liu, SL; Sun, J (28 October 2022). "Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination". Science Immunology. 7 (76): eadd4853. doi:10.1126/sciimmunol.add4853. PMC 9348751. PMID 35857583.
- Topol, EJ; Iwasaki, A (12 August 2022). "Operation Nasal Vaccine-Lightning speed to counter COVID-19". Science Immunology. 7 (74): eadd9947. doi:10.1126/sciimmunol.add9947. PMID 35862488. S2CID 250954536.
- Mao, T; Israelow, B; Peña-Hernández, MA; Suberi, A; Zhou, L; Luyten, S; Reschke, M; Dong, H; Homer, RJ; Saltzman, WM; Iwasaki, A (27 October 2022). "Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses". Science. 378 (6622): eabo2523. doi:10.1126/science.abo2523. PMC 9798903. PMID 36302057. S2CID 253182948.
- Kozlowski PA, Aldovini A (2019-04-12). "Mucosal Vaccine Approaches for Prevention of HIV and SIV Transmission". Current Immunology Reviews. 15 (1): 102–122. doi:10.2174/1573395514666180605092054. PMC 6709706. PMID 31452652.
- Wild C, Wallner M, Hufnagl K, Fuchs H, Hoffmann-Sommergruber K, Breiteneder H, et al. (January 2007). "A recombinant allergen chimer as novel mucosal vaccine candidate for prevention of multi-sensitivities". Allergy. 62 (1): 33–41. doi:10.1111/j.1398-9995.2006.01245.x. PMID 17156339. S2CID 9883901.
- Lavelle EC, Ward RW (July 2021). "Mucosal vaccines - fortifying the frontiers". Nature Reviews. Immunology. 22 (4): 236–250. doi:10.1038/s41577-021-00583-2. PMC 8312369. PMID 34312520.
Further reading
- Helbert M (2016-06-17). Immunology for Medical Students. Elsevier. ISBN 978-0-7020-6801-0.