Multiple sclerosis
Multiple sclerosis (MS) is a degenerative disease in which the insulating covers called myelin sheaths, of nerve cells in the brain and spinal cord are damaged.[3] This damage disrupts the ability of parts of the nervous system to transmit signals, resulting in a range of signs and symptoms, including physical, mental, and sometimes psychiatric problems.[1][8][9] Specific symptoms can include double vision, visual loss, muscle weakness, and trouble with sensation or coordination.[3][10][11] MS takes several forms, with new symptoms either occurring in isolated attacks (relapsing forms) or building up over time (progressive forms).[12][13] In the relapsing forms of MS, between attacks, symptoms may disappear completely, although some permanent neurological problems often remain, especially as the disease advances.[13]
| Multiple sclerosis | |
|---|---|
| Other names | Multiple cerebral sclerosis, multiple cerebro-spinal sclerosis, disseminated sclerosis, encephalomyelitis disseminata |
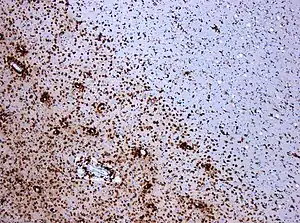 | |
| CD68-stained tissue shows several macrophages in the area of a demyelinated lesion caused by MS. | |
| Specialty | Neurology |
| Symptoms | Variable, including almost any neurological symptom or sign, with autonomic, visual, motor, and sensory problems being the most common.[1] |
| Usual onset | Age 20–50[2] |
| Duration | Long term[3] |
| Causes | Unknown[4] |
| Diagnostic method | Based on symptoms and medical tests[5] |
| Treatment | Disease-Modifying Therapies[6]
Physiotherapy[7] Occupational Therapy[7] |
| Frequency | 0.032% (world) |
While the cause is unclear, the underlying mechanism is thought to be either destruction by the immune system or failure of the myelin-producing cells.[4] Proposed causes for this include genetics and environmental factors, such as viral infections.[14][8][15] MS is usually diagnosed based on the presenting signs and symptoms and the results of supporting medical tests.[5]
No cure for multiple sclerosis is known.[3] Treatments attempt to improve function after an attack and prevent new attacks.[8] Physical therapy[7] and occupational therapy[16] can help with people's ability to function. Many people pursue alternative treatments, despite a lack of evidence of benefit.[17] The long-term outcome is difficult to predict; better outcomes are more often seen in women, those who develop the disease early in life, those with a relapsing course, and those who initially experienced few attacks.[18]
Multiple sclerosis is the most common immune-mediated disorder affecting the central nervous system.[19] Nearly one million people have MS in the United States in 2022,[20] and in 2020, about 2.8 million people were affected globally, with rates varying widely in different regions and among different populations.[21] The disease usually begins between the ages of 20 and 50 and is twice as common in women as in men.[2] MS was first described in 1868 by French neurologist Jean-Martin Charcot.[22]
The name "multiple sclerosis" is short for multiple cerebro-spinal sclerosis, which refers to the numerous glial scars (or sclerae – essentially plaques or lesions) that develop on the white matter of the brain and spinal cord.[22]
Signs and symptoms
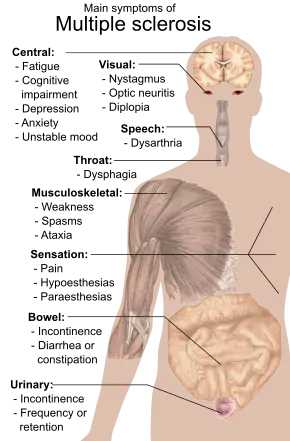
A person with MS can have almost any neurological symptom or sign, with autonomic, visual, motor, and sensory problems being the most common.[1] The specific symptoms are determined by the locations of the lesions within the nervous system, and may include loss of sensitivity or changes in sensation, such as tingling, pins and needles, or numbness; muscle weakness, blurred vision,[23] pronounced reflexes, muscle spasms, difficulty in moving, difficulties with coordination, and balance (ataxia); problems with speech or swallowing, visual problems (nystagmus, optic neuritis,[24] or double vision), feeling tired, acute or chronic pain; and bladder and bowel difficulties (such as neurogenic bladder), among others.[1] When multiple sclerosis is more advanced, walking difficulties can occur and the risk of falling increases.[25]
Difficulties thinking and emotional problems such as depression or unstable mood are also common.[1] The primary deficit in cognitive function that people with MS experience is slowed information-processing speed, with memory also commonly affected, and executive function less commonly. Intelligence, language, and semantic memory are usually preserved, and the level of cognitive impairment varies considerably between people with MS.[26][27][28]
Uhthoff's phenomenon, a worsening of symptoms due to exposure to higher-than-usual temperatures, and Lhermitte's sign, an electrical sensation that runs down the back when bending the neck, are particularly characteristic of MS.[1] The main measure of disability and severity is the expanded disability status scale (EDSS), with other measures such as the multiple sclerosis functional composite being increasingly used in research.[29][30][31] EDSS is also correlated with falls in people with MS.[10] While it is a popular measure, EDSS has been criticized for some of its limitations, such as relying too much on walking.[32][10]
The condition begins in 85% of cases as a clinically isolated syndrome (CIS) over a number of days with 45% having motor or sensory problems, 20% having optic neuritis,[24] and 10% having symptoms related to brainstem dysfunction, while the remaining 25% have more than one of the previous difficulties.[5] The course of symptoms occurs in two main patterns initially: either as episodes of sudden worsening that last a few days to months (called relapses, exacerbations, bouts, attacks, or flare-ups) followed by improvement (85% of cases) or as a gradual worsening over time without periods of recovery (10–15% of cases).[2] A combination of these two patterns may also occur[13] or people may start in a relapsing and remitting course that then becomes progressive later on.[2]
Relapses are usually not predictable, occurring without warning.[1] Exacerbations rarely occur more frequently than twice per year.[1] Some relapses, however, are preceded by common triggers and they occur more frequently during spring and summer.[33] Similarly, viral infections such as the common cold, influenza, or gastroenteritis increase their risk.[1] Stress may also trigger an attack.[34] Women with MS who become pregnant experience fewer relapses; however, during the first months after delivery the risk increases.[1] Overall, pregnancy does not seem to influence long-term disability.[1] Many events have been found not to affect relapse rates including vaccination,[35] breast feeding,[1] physical trauma,[36] and Uhthoff's phenomenon.[33]
Causes
The cause of MS is unknown, but it is believed to occur as a result of some combination of genetic and environmental factors, such as infectious agents.[1]
Infectious agents
Many microbes have been proposed as triggers of MS.[8] One hypothesis is that infection by a widespread microbe contributes to disease development, and the geographic distribution of this organism influences the epidemiology of MS.[15] Two opposing versions of this hypothesis include the hygiene hypothesis and the prevalence hypothesis, the former being more favored.[15] The hygiene hypothesis proposes that exposure to certain infectious agents early in life is protective; the disease is a response to a late encounter with such agents.[1] The prevalence hypothesis proposes that an early, persistent, and silent infection increases risk of disease, thus the disease is more common where the infectious agent is more common. Only in a few cases and after many years does it cause demyelination.[15][39] Evidence for a virus as a cause include the presence of oligoclonal bands in the brain and cerebrospinal fluid of most people with MS, the association of several viruses with human demyelinating encephalomyelitis, and the occurrence of demyelination in animals caused by some viral infections.[40]
Epstein-Barr herpes virus (EBV) can cause infectious mononucleosis and infects about 95% of adults. In combination with other genetic and environmental factors, there is "compelling epidemiological and mechanistic evidence for a causal role of EBV in multiple sclerosis", though only a small proportion of those infected with EBV later develop MS.[41][14][42] A study of individuals in the United States military between 1993 and 2013 (total population greater than 10 million) compared 801 people who developed MS on or after military service to 1,566 matched controls who did not develop MS during this observation period. The study found a 32-fold increased risk of developing MS after infection with EBV. It did not find an increased risk after infection with other viruses, including the similarly transmitted cytomegalovirus. The finding strongly suggests that EBV plays a role in the onset of MS, although EBV alone may be insufficient to cause it.[14][42]
Genetics

MS is not considered a hereditary disease, but several genetic variations have been shown to increase the risk.[43] Some of these genes appear to have higher levels of expression in microglial cells than expected by chance.[44] The probability of developing the disease is higher in relatives of an affected person, with a greater risk among those more closely related.[8] An identical twin of an affected individual has a 30% chance of developing MS, 5% for a nonidentical twin, 2.5% for a sibling, and an even lower chance for a half sibling.[1][8][45] If both parents are affected, the risk in their children is 10 times that of the general population.[2] MS is also more common in some ethnic groups than others.[46]
Specific genes that have been linked with MS include differences in the human leukocyte antigen (HLA) system—a group of genes on chromosome 6 that serves as the major histocompatibility complex (MHC).[1] That differences in the HLA region are related to susceptibility has been known since the 1980s,[47] and this same region has also been implicated in the development of other autoimmune diseases, such as diabetes type I and systemic lupus erythematosus.[47] The most consistent finding is the association between multiple sclerosis and alleles of the MHC defined as DR15 and DQ6.[1] Other loci have shown a protective effect, such as HLA-C554 and HLA-DRB1*11.[1] HLA differences account for an estimated 20 to 60% of the genetic predisposition.[47] Modern genetic methods (genome-wide association studies) have revealed at least 200 variants outside the HLA locus that modestly increase the probability of MS.[48]
Geography
MS is more common in people who live farther from the equator, although exceptions exist.[1][49] These exceptions include ethnic groups that are at low risk and that live far from the equator such as the Sami, Amerindians, Canadian Hutterites, New Zealand Māori,[50] and Canada's Inuit,[2] as well as groups that have a relatively high risk and that live closer to the equator such as Sardinians,[2] inland Sicilians,[51] Palestinians, and Parsi.[50] The cause of this geographical pattern is not clear.[2] While the north–south gradient of incidence is decreasing,[49] as of 2010 it is still present.[2]
MS is more common in regions with northern European populations,[1] so the geographic variation may simply reflect the global distribution of these high-risk populations.[2]
A relationship between season of birth and MS lends support to this idea, with fewer people born in the Northern Hemisphere in November compared to May being affected later in life.[52]
Environmental factors may play a role during childhood, with several studies finding that people who move to a different region of the world before the age of 15 acquire the new region's risk of MS. If migration takes place after age 15, the persons retain the risk of their home country.[1][53] Some evidence indicates that the effect of moving may still apply to people older than 15.[1]
Other
Smoking may be an independent risk factor for MS.[54] Stress may be a risk factor, although the evidence to support this is weak.[53] Association with occupational exposures and toxins—mainly organic solvents[55]—has been evaluated, but no clear conclusions have been reached.[53] Vaccinations were studied as causal factors; most studies, though, show no association.[53][56] Several other possible risk factors, such as diet and hormone intake, have been evaluated, but evidence on their relation with the disease is "sparse and unpersuasive".[54] Gout occurs less than would be expected and lower levels of uric acid have been found in people with MS. This has led to the theory that uric acid is protective, although its exact importance remains unknown.[57] Obesity during adolescence and young adulthood is a risk factor for MS.[58]
Pathophysiology
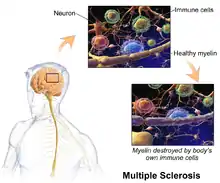
The three main characteristics of MS are the formation of lesions in the central nervous system (also called plaques), inflammation, and the destruction of myelin sheaths of neurons. These features interact in a complex and not yet fully understood manner to produce the breakdown of nerve tissue, and in turn, the signs and symptoms of the disease.[1] Cholesterol crystals are believed both to impair myelin repair and aggravate inflammation.[59][60] MS is believed to be an immune-mediated disorder that develops from an interaction of the individual's genetics and as yet unidentified environmental causes.[8] Damage is believed to be caused, at least in part, by attack on the nervous system by a person's own immune system.[1]
Lesions

The name multiple sclerosis refers to the scars (sclerae – better known as plaques or lesions) that form in the nervous system. These lesions most commonly affect the white matter in the optic nerve, brain stem, basal ganglia, and spinal cord, or white matter tracts close to the lateral ventricles.[1] The function of white matter cells is to carry signals between grey matter areas, where the processing is done, and the rest of the body. The peripheral nervous system is rarely involved.[8]
To be specific, MS involves the loss of oligodendrocytes, the cells responsible for creating and maintaining a fatty layer—known as the myelin sheath—which helps the neurons carry electrical signals (action potentials).[1] This results in a thinning or complete loss of myelin, and as the disease advances, the breakdown of the axons of neurons. When the myelin is lost, a neuron can no longer effectively conduct electrical signals.[8] A repair process, called remyelination, takes place in early phases of the disease, but the oligodendrocytes are unable to completely rebuild the cell's myelin sheath.[61] Repeated attacks lead to successively less effective remyelinations, until a scar-like plaque is built up around the damaged axons.[61] These scars are the origin of the symptoms and during an attack magnetic resonance imaging (MRI) often shows more than 10 new plaques.[1] This could indicate that some number of lesions exist, below which the brain is capable of repairing itself without producing noticeable consequences.[1] Another process involved in the creation of lesions is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons.[1] A number of lesion patterns have been described.[62]
Inflammation
Apart from demyelination, the other sign of the disease is inflammation. Fitting with an immunological explanation, the inflammatory process is caused by T cells, a kind of lymphocytes that plays an important role in the body's defenses.[8] T cells gain entry into the brain as a result of disruptions in the blood–brain barrier. The T cells recognize myelin as foreign and attack it, explaining why these cells are also called "autoreactive lymphocytes".[1]
The attack on myelin starts inflammatory processes, which trigger other immune cells and the release of soluble factors like cytokines and antibodies. A further breakdown of the blood-brain barrier, in turn, causes a number of other damaging effects, such as swelling, activation of macrophages, and more activation of cytokines and other destructive proteins.[8] Inflammation can potentially reduce transmission of information between neurons in at least three ways.[1] The soluble factors released might stop neurotransmission by intact neurons. These factors could lead to or enhance the loss of myelin, or they may cause the axon to break down completely.[1]
Blood–brain barrier
The blood–brain barrier (BBB) is a part of the capillary system that prevents the entry of T cells into the central nervous system. It may become permeable to these types of cells secondary to an infection by a virus or bacteria. After it repairs itself, typically once the infection has cleared, T cells may remain trapped inside the brain.[8][63] Gadolinium cannot cross a normal BBB, so gadolinium-enhanced MRI is used to show BBB breakdowns.[64]
Diagnosis

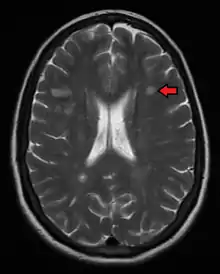
Multiple sclerosis is typically diagnosed based on the presenting signs and symptoms, in combination with supporting medical imaging and laboratory testing.[5] It can be difficult to confirm, especially early on, since the signs and symptoms may be similar to those of other medical problems.[1][65]
The McDonald criteria, which focus on clinical, laboratory, and radiologic evidence of lesions at different times and in different areas, is the most commonly used method of diagnosis[66] with the Schumacher and Poser criteria being of mostly historical significance.[67] The McDonald criteria states that patients with multiple sclerosis should have lesions which are disseminated in time (DIT) and disseminated in space (DIS), i.e. lesions which have appeared in different areas in the brain and at different times.
As of 2017, no single test (including biopsy) can provide a definitive diagnosis.[68]
Magnetic resonance imaging (MRI) of the brain and spine may show areas of demyelination (lesions or plaques). Gadolinium can be administered intravenously as a contrast agent to highlight active plaques, and by elimination, demonstrate the existence of historical lesions not associated with symptoms at the moment of the evaluation.[69][70]
Central vein signs (CVSs) have been proposed as a good indicator of MS in comparison with other conditions causing white lesions.[71][72][73][74] One small study found fewer CVSs in older and hypertensive people.[75] Further research on CVS as a biomarker for MS is ongoing.[76]
Brain atrophy is seen as an indicator of MS.[77][78]
Testing of cerebrospinal fluid obtained from a lumbar puncture can provide evidence of chronic inflammation in the central nervous system. The cerebrospinal fluid is tested for oligoclonal bands of IgG on electrophoresis, which are inflammation markers found in 75–85% of people with MS.[69][79]
Differential diagnosis
Several diseases present similarly to MS.[80][81] Medical professionals use a patient's specific presentation, history, and exam findings to make an individualized differential. Red flags are findings that suggest an alternate diagnosis, although they do not rule out MS. Red flags include a patient younger than 15 or older than 60, less than 24 hours of symptoms, involvement of multiple cranial nerves, involvement of organs outside of the nervous system, and atypical lab and exam findings.[80][81]
In an emergency setting, it is important to rule out a stroke or bleeding in the brain.[81] Intractable vomiting, severe optic neuritis,[24] or bilateral optic neuritis[24] raises suspicion for neuromyelitis optica spectrum disorder (NMOSD).[82] Infectious diseases that may look similar to multiple sclerosis include HIV, Lyme disease, and Syphilis. Autoimmune diseases include Sarcoidosis, Lupus, Guillain-Barré syndrome, Acute disseminated encephalomyelitis, and Behçet's disease. Psychiatric conditions such as Anxiety or Conversion disorder may also present in a similar way. Other rare diseases on the differential include CNS lymphoma, congenital leukodystrophies, and anti-MOG-associated myelitis.[80][81]
Types and variants
Several phenotypes (commonly termed "types"), or patterns of progression, have been described. Phenotypes use the past course of the disease in an attempt to predict the future course. They are important not only for prognosis, but also for treatment decisions.
The International Advisory Committee on Clinical Trials of MS describes four types of MS (revised in 2013) in what is known as the Lublin classification:[83][84]
- Clinically isolated syndrome (CIS)
- Relapsing-remitting MS (RRMS)
- Primary progressive MS (PPMS)
- Secondary progressive MS (SPMS)
CIS can be characterised as a single lesion seen on MRI which is associated with signs and/or symptoms found in MS. Due to the McDonald criteria, it does not completely fit the criteria to be diagnosed as MS, hence being named "clinically isolated syndrome". CIS can be seen as the first episode of demyelination in the central nervous system. To be classified as CIS, the attack must last at least 24 hours and be caused by inflammation or demyelination of the central nervous system.[1][85] Patients who suffer from CIS may or may not go on to develop MS, but 30 to 70% of persons who experience CIS will later develop MS.[86]
RRMS is characterized by unpredictable relapses followed by periods of months to years of relative quiet (remission) with no new signs of disease activity. Deficits that occur during attacks may either resolve or leave problems, the latter in about 40% of attacks and being more common the longer a person has had the disease.[1][5] This describes the initial course of 80% of individuals with MS.[1]
PPMS occurs in roughly 10–20% of individuals with the disease, with no remission after the initial symptoms.[5][87] It is characterized by progression of disability from onset, with no, or only occasional and minor, remissions and improvements.[13] The usual age of onset for the primary progressive subtype is later than of the relapsing-remitting subtype. It is similar to the age that secondary progressive usually begins in RRMS, around 40 years of age.[1]
SPMS occurs in around 65% of those with initial RRMS, who eventually have progressive neurologic decline between acute attacks without any definite periods of remission.[1][13] Occasional relapses and minor remissions may appear.[13] The most common length of time between disease onset and conversion from RRMS to SPMS is 19 years.[88]
Special courses
Independently of the types published by the MS associations, regulatory agencies such as the FDA often consider special courses, trying to reflect some clinical trials results on their approval documents. Some examples could be "highly active MS" (HAMS),[89] "active secondary MS" (similar to the old progressive-relapsing)[90] and "rapidly progressing PPMS".[91]
Also, deficits always resolving between attacks is sometimes referred to as "benign" MS,[92] although people still build up some degree of disability in the long term.[1] On the other hand, the term malignant multiple sclerosis is used to describe people with MS having reached significant level of disability in a short period.[93]
An international panel has published a standardized definition for the course HAMS.[89]
Variants
Atypical variants of MS have been described; these include tumefactive multiple sclerosis, Balo concentric sclerosis, Schilder's diffuse sclerosis, and Marburg multiple sclerosis. Debate remains on whether they are MS variants or different diseases.[94] Some diseases previously considered MS variants, such as Devic's disease, are now considered outside the MS spectrum.[95]
Management
Although no cure for multiple sclerosis has been found, several therapies have proven helpful. Several effective treatments can decrease the number of attacks and the rate of progression.[20] The primary aims of therapy are returning function after an attack, preventing new attacks, and preventing disability. Starting medications is generally recommended in people after the first attack when more than two lesions are seen on MRI.[96]
The first approved medications used to treat MS were modestly effective, though were poorly tolerated and had many adverse effects.[3] Several treatment options with better safety and tolerability profiles have been introduced,[20] improving the prognosis of MS.
As with any medical treatment, medications used in the management of MS have several adverse effects. Alternative treatments are pursued by some people, despite the shortage of supporting evidence of efficacy.
Initial management of acute flare
During symptomatic attacks, administration of high doses of intravenous corticosteroids, such as methylprednisolone, is the usual therapy,[1] with oral corticosteroids seeming to have a similar efficacy and safety profile.[97] Although effective in the short term for relieving symptoms, corticosteroid treatments do not appear to have a significant impact on long-term recovery.[98][99] The long-term benefit is unclear in optic neuritis as of 2020.[100][24] The consequences of severe attacks that do not respond to corticosteroids might be treatable by plasmapheresis.[1]
Relapsing remitting multiple sclerosis
Multiple disease-modifying medications were approved by regulatory agencies for RRMS; they are modestly effective at decreasing the number of attacks.[101] Interferons[102] and glatiramer acetate are first-line treatments[5] and are roughly equivalent, reducing relapses by approximately 30%.[103] Early-initiated long-term therapy is safe and improves outcomes.[104][105]
Treatment of CIS with interferons decreases the chance of progressing to clinical MS.[1][106][107] Efficacy of interferons and glatiramer acetate in children has been estimated to be roughly equivalent to that of adults.[108] The role of some newer agents such as fingolimod,[109] teriflunomide, and dimethyl fumarate,[110] is not yet entirely clear.[111] Making firm conclusions about the best treatment is difficult, especially regarding the long‐term benefit and safety of early treatment, given the lack of studies directly comparing disease-modifying therapies or long-term monitoring of patient outcomes.[112]
The relative effectiveness of different treatments is unclear, as most have only been compared to placebo or a small number of other therapies.[113] Direct comparisons of interferons and glatiramer acetate indicate similar effects or only small differences in effects on relapse rate, disease progression, and MRI measures.[114] Alemtuzumab, natalizumab, and fingolimod may be more effective than other drugs in reducing relapses over the short term in people with RRMS.[115] Natalizumab and interferon beta-1a (Rebif) may reduce relapses compared to both placebo and interferon beta-1a (Avonex) while Interferon beta-1b (Betaseron), glatiramer acetate, and mitoxantrone may also prevent relapses.[113] Evidence on relative effectiveness in reducing disability progression is unclear.[113][115] All medications are associated with adverse effects that may influence their risk to benefit profiles.[113][115]
Ublituximab was approved for medical use in the United States in December 2022.[116]
Progressive multiple sclerosis
In 2011, mitoxantrone was the first medication approved for secondary progressive MS.[117] In this population, tentative evidence supports mitoxantrone moderately slowing the progression of the disease and decreasing rates of relapses over two years.[118][119]
New approved medications continue to emerge in modern medicine. In March 2017, the FDA approved ocrelizumab as a treatment for primary progressive MS in adults, the first drug to gain that approval,[120][121][122] with requirements for several Phase IV clinical trials.[123] It is also used for the treatment of relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease in adults.[122] According to a 2021 Cochrane review, ocrelizumab may reduce worsening of symptoms for primary progressive MS and probably increases unwanted effects but makes little or no difference to the number of serious unwanted effects.[124]
In 2019, siponimod and cladribine were approved in the United States for the treatment of secondary progressive multiple sclerosis (SPMS).[120] Subsequently, ozanimod was approved in 2020, and ponesimod was approved in 2021, which were both approved for management of CIS, relapsing MS, and SPMS in the U.S., and RRMS in Europe.[125]
Adverse effects
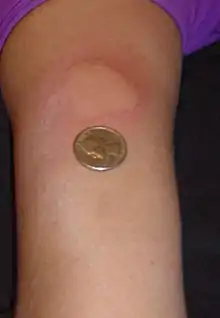
The disease-modifying treatments have several adverse effects. One of the most common is irritation at the injection site for glatiramer acetate and the interferons (up to 90% with subcutaneous injections and 33% with intramuscular injections).[102][126] Over time, a visible dent at the injection site, due to the local destruction of fat tissue, known as lipoatrophy, may develop.[126] Interferons may produce flu-like symptoms;[127] some people taking glatiramer experience a post-injection reaction with flushing, chest tightness, heart palpitations, and anxiety, which usually lasts less than thirty minutes.[128] More dangerous but much less common are liver damage from interferons,[129] systolic dysfunction (12%), infertility, and acute myeloid leukemia (0.8%) from mitoxantrone,[118][130] and progressive multifocal leukoencephalopathy occurring with natalizumab (occurring in 1 in 600 people treated).[5][131]
Fingolimod may give rise to hypertension and slowed heart rate, macular edema, elevated liver enzymes, or a reduction in lymphocyte levels.[109][111] Tentative evidence supports the short-term safety of teriflunomide, with common side effects including: headaches, fatigue, nausea, hair loss, and limb pain.[101] There have also been reports of liver failure and PML with its use and it is dangerous for fetal development.[111] Most common side effects of dimethyl fumarate are flushing and gastrointestinal problems.[110][132][111] While dimethyl fumarate may lead to a reduction in the white blood cell count there were no reported cases of opportunistic infections during trials.[133]
Associated symptoms
Both medications and neurorehabilitation have been shown to improve some symptoms, though neither changes the course of the disease.[134] Some symptoms have a good response to medication, such as bladder spasticity, while others are little changed.[1] Equipment such as catheters for neurogenic bladder dysfunction or mobility aids can be helpful in improving functional status.
A multidisciplinary approach is important for improving quality of life; however, it is difficult to specify a 'core team' as many health services may be needed at different points in time.[1] Multidisciplinary rehabilitation programs increase activity and participation of people with MS but do not influence impairment level.[135] Studies investigating information provision in support of patient understanding and participation suggest that while interventions (written information, decision aids, coaching, educational programmes) may increase knowledge, the evidence of an effect on decision making and quality of life is mixed and low certainty.[136] There is limited evidence for the overall efficacy of individual therapeutic disciplines,[137][138] though there is good evidence that specific approaches, such as exercise,[139][140][141][142] and psychological therapies are effective.[143] Cognitive training, alone or combined with other neuropsychological interventions, may show positive effects for memory and attention though firm conclusions are not possible given small sample numbers, variable methodology, interventions and outcome measures.[144] The effectiveness of palliative approaches in addition to standard care is uncertain, due to lack of evidence.[145] The effectiveness of interventions, including exercise, specifically for the prevention of falls in people with MS is uncertain, while there is some evidence of an effect on balance function and mobility.[146] Cognitive behavioral therapy has shown to be moderately effective for reducing MS fatigue.[147] The evidence for the effectiveness of non-pharmacological interventions for chronic pain is insufficient to recommend such interventions alone, however their use in combination with medications may be reasonable.[148]
Non-pharmaceutical
There is some evidence that aquatic therapy is a beneficial intervention.[149]
The spasticity associated with MS can be difficult to manage because of the progressive and fluctuating course of the disease.[150] Although there is no firm conclusion on the efficacy in reducing spasticity, PT interventions can be a safe and beneficial option for patients with multiple sclerosis. Physical therapy including vibration interventions, electrical stimulation, exercise therapy, standing therapy, and radial shock wave therapy (RSWT), were beneficial for limiting spasticity, helping limit excitability, or increasing range of motion.[151]
Alternative treatments
Over 50% of people with MS may use complementary and alternative medicine, although percentages vary depending on how alternative medicine is defined.[17] Regarding the characteristics of users, they are more frequently women, have had MS for a longer time, tend to be more disabled and have lower levels of satisfaction with conventional healthcare.[17] The evidence for the effectiveness for such treatments in most cases is weak or absent.[17][152] Treatments of unproven benefit used by people with MS include dietary supplementation and regimens,[17][153][154] vitamin D,[155] relaxation techniques such as yoga,[17] herbal medicine (including medical cannabis),[17][156][157] hyperbaric oxygen therapy,[158] self-infection with hookworms, reflexology, acupuncture,[17][159] and mindfulness.[160] Evidence suggests vitamin D supplementation, irrespective of the form and dose, provides no benefit for people with MS; this includes for measures such as relapse recurrence, disability, and MRI lesions while effects on health‐related quality of life and fatigue are unclear.[161] There is insufficient evidence supporting high-dose biotin[162][163][164] and some evidence for increased disease activity and higher risk of relapse with its use.[165]
Prognosis
The availability of treatments that modify the course of multiple sclerosis beginning in the 1990s, known as disease-modifying therapies (DMTs), has improved prognosis. These treatments can reduce relapses and slow progression, but as of 2022 there is no cure.[20][166]
The prognosis of MS depends on the subtype of the disease, and there is considerable individual variation in the progression of the disease.[167] In relapsing MS, the most common subtype, a 2016 cohort study found that after a median of 16.8 years from onset, one in ten needed a walking aid, and almost two in ten transitioned to secondary progressive MS, a form characterized by more progressive decline.[20] With treatments available in the 2020s, relapses can be eliminated or substantially reduced. However, "silent progression" of the disease still occurs.[166][168]
In addition to secondary progressive MS (SPMS), a small proportion of people with MS (10–15%) experience progressive decline from the onset, known as primary progressive MS (PPMS). Most treatments have been approved for use in relapsing MS; there are fewer treatments with lower efficacy for progressive forms of MS.[169][166][20] The prognosis for progressive MS is worse, with faster accumulation of disability, though with considerable individual variation.[169] In untreated PPMS, the median time from onset to requiring a walking aid is estimated as seven years.[20] In SPMS, a 2014 cohort study reported that people required a walking aid after an average of five years from onset of SPMS, and were chair or bed-bound after an average of fifteen years.[170]
After diagnosis of MS, characteristics that predict a worse course are male sex, older age, and greater disability at the time of diagnosis; female sex is associated with a higher relapse rate.[171] As of 2018, no biomarker can accurately predict disease progression in every patient.[167] Spinal cord lesions, abnormalities on MRI, and more brain atrophy are predictive of a worse course, though brain atrophy as a predictor of disease course is experimental and not used in clinical practice as of 2018.[171] Early treatment leads to a better prognosis, but a higher relapse frequency when treated with DMTs is associated with a poorer prognosis.[167][171] A 60-year longitudinal population study conducted in Norway found a 7-year shorter life expectancy in MS compared with the general population and a rise in survival in MS during the observation period. Median life expectancy for RRMS patients was 77.8 years and 71.4 years for PPMS, compared to 81.8 years for the general population. Life expectancy for men was 5 years shorter than for women.[172]
Epidemiology

MS is the most common autoimmune disorder of the central nervous system.[19] The latest estimation of the total number of people with MS was 2.8 million globally, with a prevalence of 36 per 100,000 people. Moreover, prevalence varies widely in different regions around the world.[21] In Africa, there are 5 people per 100,000 diagnosed with MS, compared to South East Asia where the prevalence is 9 per 100,000, 112 per 100,000 in the Americas, and 133 per 100,000 in Europe.[173]
Increasing rates of MS may be explained simply by better diagnosis.[2] Studies on populational and geographical patterns have been common[39] and have led to a number of theories about the cause.[15][53][54]
MS usually appears in adults in their late twenties or early thirties but it can rarely start in childhood and after 50 years of age.[2][66] The primary progressive subtype is more common in people in their fifties.[87] Similarly to many autoimmune disorders, the disease is more common in women, and the trend may be increasing.[1][49] As of 2020, globally it is about two times more common in women than in men, and the ratio of women to men with MS is as high as 4:1 in some countries.[174] In children, it is even more common in females than males,[1] while in people over fifty, it affects males and females almost equally.[87]
History
Medical discovery
Robert Carswell (1793–1857), a British professor of pathology, and Jean Cruveilhier (1791–1873), a French professor of pathologic anatomy, described and illustrated many of the disease's clinical details, but did not identify it as a separate disease.[175] Specifically, Carswell described the injuries he found as "a remarkable lesion of the spinal cord accompanied with atrophy".[1] Under the microscope, Swiss pathologist Georg Eduard Rindfleisch (1836–1908) noted in 1863 that the inflammation-associated lesions were distributed around blood vessels.[176][177]
The French neurologist Jean-Martin Charcot (1825–1893) was the first person to recognize multiple sclerosis as a distinct disease in 1868.[175] Summarizing previous reports and adding his own clinical and pathological observations, Charcot called the disease sclerose en plaques.
Diagnosis history
The first attempt to establish a set of diagnostic criteria was also due to Charcot in 1868. He published what now is known as the "Charcot Triad", consisting in nystagmus, intention tremor, and telegraphic speech (scanning speech).[178] Charcot also observed cognition changes, describing his patients as having a "marked enfeeblement of the memory" and "conceptions that formed slowly".[22]
Diagnosis was based on Charcot triad and clinical observation until Schumacher made the first attempt to standardize criteria in 1965 by introducing some fundamental requirements: Dissemination of the lesions in time (DIT) and space (DIS), and that "signs and symptoms cannot be explained better by another disease process".[178] The DIT and DIS requirement was later inherited by the Poser and McDonald criteria, whose 2017 revision is in use.[178][167]
During the 20th century, theories about the cause and pathogenesis were developed and effective treatments began to appear in the 1990s.[1] Since the beginning of the 21st century, refinements of the concepts have taken place. The 2010 revision of the McDonald criteria allowed for the diagnosis of MS with only one proved lesion (CIS).[179]
In 1996, the US National Multiple Sclerosis Society (NMSS) (Advisory Committee on Clinical Trials) defined the first version of the clinical phenotypes that is in use. In this first version they provided standardized definitions for four MS clinical courses: relapsing-remitting (RR), secondary progressive (SP), primary progressive (PP), and progressive relapsing (PR). In 2010, PR was dropped and CIS was incorporated.[179] Three years later, the 2013 revision of the "phenotypes for the disease course" were forced to consider CIS as one of the phenotypes of MS, making obsolete some expressions like "conversion from CIS to MS".[180] Other organizations have proposed later new clinical phenotypes, like HAMS (Highly Active MS).[181]
Historical cases
.jpg.webp)
There are several historical accounts of people who probably had MS and lived before or shortly after the disease was described by Charcot.
A young woman called Halldora who lived in Iceland around 1200 suddenly lost her vision and mobility but recovered them seven days after. Saint Lidwina of Schiedam (1380–1433), a Dutch nun, may be one of the first clearly identifiable people with MS. From the age of 16 until her death at 53, she had intermittent pain, weakness of the legs and vision loss: symptoms typical of MS.[182] Both cases have led to the proposal of a "Viking gene" hypothesis for the dissemination of the disease.[183]
Augustus Frederick d'Este (1794–1848), son of Prince Augustus Frederick, Duke of Sussex and Lady Augusta Murray and a grandson of George III of the United Kingdom, almost certainly had MS. D'Este left a detailed diary describing his 22 years living with the disease. His diary began in 1822 and ended in 1846, although it remained unknown until 1948. His symptoms began at age 28 with a sudden transient visual loss (amaurosis fugax) after the funeral of a friend. During his disease, he developed weakness of the legs, clumsiness of the hands, numbness, dizziness, bladder disturbance and erectile dysfunction. In 1844, he began to use a wheelchair. Despite his illness, he kept an optimistic view of life.[184][185] Another early account of MS was kept by the British diarist W. N. P. Barbellion, pen name of Bruce Frederick Cummings (1889–1919), who maintained a detailed log of his diagnosis and struggle.[185] His diary was published in 1919 as The Journal of a Disappointed Man.[186] Charles Dickens, a keen observer, described possible bilateral optic neuritis with reduced contrast vision and Uhthoff phenomenon in the main female character of Bleak House (1852–1853), Esther Summerville.[187]
Research
Epstein-Barr virus
As of 2022, the pathogenesis of MS as it relates to Epstein-Barr virus (EBV) is actively investigated, as are disease-modifying therapies; understanding of how risk factors combine with EBV to initiate MS is sought. Whether EBV is the only cause of MS might be better understood if an EBV vaccine is developed and shown to prevent MS as well.[14]
Even though a variety of studies showed the connection between an EBV infection and a later development of multiple sclerosis, the mechanisms behind this correlation are not completely clear, and several theories has been proposed to explain the relationship between the two diseases. It is thought that the involvement of EBV-infected B-cells[188] and the involvement of anti-EBNA antibodies, which appear to be significantly higher in multiple sclerosis patients, play a crucial role in the development of the disease.[189] This is supported by the fact that treatment against B-cells, e.g. ocrelizumab, reduces the symptoms of multiple sclerosis: annual relapses appear less frequently and the disability progression is slower.[190] A 2022 Stanford University study has shown that during an EBV infection, molecular mimicry can occur, where the immune system will produce antibodies against the EBNA1 protein, which at the same time is able to bind to GlialCAM in the myelin. Additionally, they observed a phenomenon which is uncommon in healthy individuals but often detected in multiple sclerosis patients – B-cells are trafficking to the brain and spinal cord, where they are producing oligoclonal antibody bands. A majority of these oligoclonal bands do have an affinity to the viral protein EBNA1, which is cross-reactive to GlialCAM. These antibodies are abundant in approximately 20–25% of multiple sclerosis patients and worsen the autoimmune demyelination which leads consequently to an pathophysiologocal exacerbation of the disease. Furthermore, the intrathecal oligoclonal expansion with a constant somatic hypermutation is unique in multiple sclerosis when compared to other neuroinflammatory diseases. In the study there was also the abundance of antibodies with IGHV 3–7 genes measured, which appears to be connected to the disease progress. Antibodies which are IGHV3–7-based are binding with a high affinity to EBNA1 and GlialCAM. This process is actively thriving the demyelination. It is probable that B-cells, expressing IGHV 3–7 genes entered the CSF and underwent affinity maturation after facing GlialCAM, which led consequently to the production of high affinity anti-GlialCAM antibodies. This was additionally shown in the EAE mouse model where immunization with EBNA1 lead to a strong B-cell response against GlialCAM, which worsened the EAE.[191]
Human endogenous retroviruses
Two members of the human endogenous retroviruses-W (HERV-W) family, namely, ERVWE1 and MS-associated retrovirus (MSRV), may be co-factors in MS immunopathogenesis. HERVs constitute up to 8% of the human genome; most are epignetically silent, but can be reactivated by exogenous viruses, proinflammatory conditions and/or oxidative stress.[192][193][194]
Medications
Medications that influence voltage-gated sodium ion channels are under investigation as a potential neuroprotective strategy because of hypothesized role of sodium in the pathological process leading to axonal injury and accumulating disability. There is insufficient evidence of an effect of sodium channel blockers for people with MS.[195]
Pathogenesis
MS is a clinically defined entity with several atypical presentations. Some auto-antibodies have been found in atypical MS cases, giving birth to separate disease families and restricting the previously wider concept of MS.
Anti-AQP4 autoantibodies were found in neuromyelitis optica (NMO), which was previously considered a MS variant. A spectrum of diseases named NMOSD (NMO spectrum diseases) or anti-AQP4 diseases has been accepted.[196] Some cases of MS were presenting anti-MOG autoantibodies, mainly overlapping with the Marburg variant. Anti-MOG autoantibodies were found to be also present in ADEM, and a second spectrum of separated diseases is being considered. This spectrum is named inconsistently across different authors, but it is normally something similar to anti-MOG demyelinating diseases.[196]
A third kind of auto-antibodies is accepted. They are several anti-neurofascin auto-antibodies which damage the Ranvier nodes of the neurons. These antibodies are more related to the peripheral nervous demyelination, but they were also found in chronic progressive PPMS and combined central and peripheral demyelination (CCPD, which is considered another atypical MS presentation).[197]
In addition to the significance of auto-antibodies in MS, four different patterns of demyelination have been reported, opening the door to consider MS as a heterogeneous disease.[198]
Disease biomarkers
Since disease progression is the result of degeneration of neurons, the roles of proteins showing loss of nerve tissue such as neurofilaments, tau, and N-acetylaspartate are under investigation.[200][201]
Improvement in neuroimaging techniques such as positron emission tomography (PET) or MRI carry a promise for better diagnosis and prognosis predictions. Regarding MRI, there are several techniques that have already shown some usefulness in research settings and could be introduced into clinical practice, such as double-inversion recovery sequences, magnetization transfer, diffusion tensor, and functional magnetic resonance imaging.[202] These techniques are more specific for the disease than existing ones, but still lack some standardization of acquisition protocols and the creation of normative values.[202] This is particularly the case for proton magnetic resonance spectroscopy, for which a number of methodological variations observed in the literature may underlie continued inconsistencies in central nervous system metabolic abnormalities, particularly in N-acetyl aspartate, myoinositol, choline, glutamate, GABA, and GSH, observed for multiple sclerosis and its subtypes.[203] There are other techniques under development that include contrast agents capable of measuring levels of peripheral macrophages, inflammation, or neuronal dysfunction,[202] and techniques that measure iron deposition that could serve to determine the role of this feature in MS, or that of cerebral perfusion.[202]
COVID-19
The hospitalization rate was found to be higher among individuals with MS and COVID-19 infection, at 10%, while the pooled infection rate is estimated at 4%. The pooled prevalence of death in hospitalized individuals with MS is estimated as 4%.[204]
Other emerging theories
One emerging hypothesis, referred to as the hygiene hypothesis, suggests that early-life exposure to infectious agents helps to develop the immune system and reduces susceptibility to allergies and autoimmune disorders, including MS. Germ-free mice infected with transplanted fecal matter from MS patients exhibit an increased risk of developing EAE, an animal model of MS.[205] It has also been proposed that certain bacteria found in the gut use molecular mimicry to infiltrate the brain via the gut-brain axis, initiating an inflammatory response and increasing blood-brain barrier permeability. Vitamin D levels have also been correlated with MS; lower levels of vitamin D correspond to an increased risk of MS, suggesting a reduced prevalence in the tropics – an area with more Vitamin D-rich sunlight – strengthening the impact of geographical location on MS development.[206] MS mechanisms begin when peripheral autoreactive effector CD4+ T cells get activated and move into the CNS. Antigen-presenting cells localize the reactivation of autoreactive effector CD4-T cells once they have entered the CNS, attracting more T cells and macrophages to form the inflammatory lesion.[207] In MS patients, macrophages and microglia assemble at locations where demyelination and neurodegeneration are actively occurring, and microglial activation is more apparent in the normal-appearing white matter of MS patients.[208] Astrocytes generate neurotoxic chemicals like nitric oxide and TNFα, attract neurotoxic inflammatory monocytes to the CNS, and are responsible for astrogliosis, the scarring that prevents the spread of neuroinflammation and kills neurons inside the scarred area.[209]
References
- Compston A, Coles A (October 2008). "Multiple sclerosis". Lancet. 372 (9648): 1502–1517. doi:10.1016/S0140-6736(08)61620-7. PMID 18970977. S2CID 195686659.
- Milo R, Kahana E (March 2010). "Multiple sclerosis: geoepidemiology, genetics and the environment". Autoimmunity Reviews. 9 (5): A387-94. doi:10.1016/j.autrev.2009.11.010. PMID 19932200.
- "NINDS Multiple Sclerosis Information Page". National Institute of Neurological Disorders and Stroke. 19 November 2015. Archived from the original on 13 February 2016. Retrieved 6 March 2016.
- Nakahara J, Maeda M, Aiso S, Suzuki N (February 2012). "Current concepts in multiple sclerosis: autoimmunity versus oligodendrogliopathy". Clinical Reviews in Allergy & Immunology. 42 (1): 26–34. doi:10.1007/s12016-011-8287-6. PMID 22189514. S2CID 21058811.
- Tsang BK, Macdonell R (December 2011). "Multiple sclerosis- diagnosis, management and prognosis". Australian Family Physician. 40 (12): 948–55. PMID 22146321. Archived from the original on 5 October 2021. Retrieved 5 October 2021.
- Liu Z, Liao Q, Wen H, Zhang Y (June 2021). "Disease modifying therapies in relapsing-remitting multiple sclerosis: A systematic review and network meta-analysis". Autoimmunity Reviews. 20 (6): 102826. doi:10.1016/j.autrev.2021.102826. PMID 33878488. S2CID 233325057.
- Alphonsus KB, Su Y, D'Arcy C (April 2019). "The effect of exercise, yoga and physiotherapy on the quality of life of people with multiple sclerosis: Systematic review and meta-analysis". Complementary Therapies in Medicine. 43: 188–195. doi:10.1016/j.ctim.2019.02.010. PMID 30935529. S2CID 86669723.
- Compston A, Coles A (April 2002). "Multiple sclerosis". Lancet. 359 (9313): 1221–1231. doi:10.1016/S0140-6736(02)08220-X. PMID 11955556. S2CID 14207583.
- Murray ED, Buttner EA, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Daroff R, Fenichel G, Jankovic J, Mazziotta J (eds.). Bradley's neurology in clinical practice (6th ed.). Philadelphia, PA: Elsevier/Saunders. ISBN 978-1-4377-0434-1.
- Piryonesi SM, Rostampour S, Piryonesi SA (April 2021). "Predicting falls and injuries in people with multiple sclerosis using machine learning algorithms". Multiple Sclerosis and Related Disorders. 49: 102740. doi:10.1016/j.msard.2021.102740. PMID 33450500. S2CID 231624230.
- Mazumder R, Murchison C, Bourdette D, Cameron M (25 September 2014). "Falls in people with multiple sclerosis compared with falls in healthy controls". PLOS ONE. 9 (9): e107620. Bibcode:2014PLoSO...9j7620M. doi:10.1371/journal.pone.0107620. PMC 4177842. PMID 25254633.
- Baecher-Allan C, Kaskow BJ, Weiner HL (February 2018). "Multiple Sclerosis: Mechanisms and Immunotherapy". Neuron. 97 (4): 742–768. doi:10.1016/j.neuron.2018.01.021. PMID 29470968. S2CID 3499974.
- Lublin FD, Reingold SC (April 1996). "Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis". Neurology. 46 (4): 907–911. doi:10.1212/WNL.46.4.907. PMID 8780061. S2CID 40213123.
- Aloisi F, Cross AH (October 2022). "MINI-review of Epstein-Barr virus involvement in multiple sclerosis etiology and pathogenesis". Journal of Neuroimmunology. 371: 577935. doi:10.1016/j.jneuroim.2022.577935. PMID 35931008. S2CID 251152784.
- Ascherio A, Munger KL (April 2007). "Environmental risk factors for multiple sclerosis. Part I: the role of infection". Annals of Neurology. 61 (4): 288–99. doi:10.1002/ana.21117. PMID 17444504. S2CID 7682774.
- Quinn É, Hynes SM (July 2021). "Occupational therapy interventions for multiple sclerosis: A scoping review". Scandinavian Journal of Occupational Therapy. 28 (5): 399–414. doi:10.1080/11038128.2020.1786160. hdl:10379/16066. PMID 32643486. S2CID 220436640.
- Huntley A (January 2006). "A review of the evidence for efficacy of complementary and alternative medicines in MS". International MS Journal. 13 (1): 5–12, 4. PMID 16420779.
- Weinshenker BG (1994). "Natural history of multiple sclerosis". Annals of Neurology. 36 (Suppl): S6-11. doi:10.1002/ana.410360704. PMID 8017890. S2CID 7140070.
- Berer K, Krishnamoorthy G (November 2014). "Microbial view of central nervous system autoimmunity". FEBS Letters. 588 (22): 4207–13. doi:10.1016/j.febslet.2014.04.007. PMID 24746689. S2CID 2772656.
- McGinley MP, Goldschmidt CH, Rae-Grant AD (February 2021). "Diagnosis and Treatment of Multiple Sclerosis: A Review". JAMA. 325 (8): 765–779. doi:10.1001/jama.2020.26858. PMID 33620411. S2CID 232019589.
- Lane J, Ng HS, Poyser C, Lucas RM, Tremlett H (July 2022). "Multiple sclerosis incidence: A systematic review of change over time by geographical region". Mult Scler Relat Disord. 63: 103932. doi:10.1016/j.msard.2022.103932. PMID 35667315. S2CID 249188137.
- Clanet M (June 2008). "Jean-Martin Charcot. 1825 to 1893". International MS Journal. 15 (2): 59–61. PMID 18782501. Archived from the original (PDF) on 30 March 2019. Retrieved 21 October 2010.
* Charcot J (1868). "Histologie de la sclerose en plaques". Gazette des Hopitaux, Paris. 41: 554–5. - "MS Signs". Webmd. Archived from the original on 30 September 2016. Retrieved 7 October 2016.
- Petzold A, Fraser CL, Abegg M, Alroughani R, Alshowaeir D, Alvarenga R, et al. (December 2022). "Diagnosis and classification of optic neuritis". The Lancet. Neurology. 21 (12): 1120–1134. doi:10.1016/s1474-4422(22)00200-9. PMID 36179757. S2CID 252564095.
- Cameron MH, Nilsagard Y (2018). "Balance, gait, and falls in multiple sclerosis". Balance, Gait, and Falls. Handbook of Clinical Neurology. Vol. 159. pp. 237–250. doi:10.1016/b978-0-444-63916-5.00015-x. ISBN 978-0-444-63916-5. PMID 30482317.
- Oreja-Guevara C, Ayuso Blanco T, Brieva Ruiz L, Hernández Pérez MÁ, Meca-Lallana V, Ramió-Torrentà L (2019). "Cognitive Dysfunctions and Assessments in Multiple Sclerosis". Frontiers in Neurology. 10: 581. doi:10.3389/fneur.2019.00581. PMC 6558141. PMID 31214113.
- Kalb R, Beier M, Benedict RH, Charvet L, Costello K, Feinstein A, et al. (November 2018). "Recommendations for cognitive screening and management in multiple sclerosis care". Multiple Sclerosis. 24 (13): 1665–1680. doi:10.1177/1352458518803785. PMC 6238181. PMID 30303036.
- Benedict RH, Amato MP, DeLuca J, Geurts JJ (October 2020). "Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues". The Lancet. Neurology. 19 (10): 860–871. doi:10.1016/S1474-4422(20)30277-5. PMC 10011205. PMID 32949546. S2CID 221744328.
- Kurtzke JF (November 1983). "Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS)". Neurology. 33 (11): 1444–52. doi:10.1212/WNL.33.11.1444. PMID 6685237.
- Amato MP, Ponziani G (August 1999). "Quantification of impairment in MS: discussion of the scales in use". Multiple Sclerosis. 5 (4): 216–9. doi:10.1191/135245899678846113. PMID 10467378.
- Rudick RA, Cutter G, Reingold S (October 2002). "The multiple sclerosis functional composite: a new clinical outcome measure for multiple sderosis trials". Multiple Sclerosis. 8 (5): 359–65. doi:10.1191/1352458502ms845oa. PMID 12356200. S2CID 31529508.
- van Munster CE, Uitdehaag BM (March 2017). "Outcome Measures in Clinical Trials for Multiple Sclerosis". CNS Drugs. 31 (3): 217–236. doi:10.1007/s40263-017-0412-5. PMC 5336539. PMID 28185158.
- Tataru N, Vidal C, Decavel P, Berger E, Rumbach L (2006). "Limited impact of the summer heat wave in France (2003) on hospital admissions and relapses for multiple sclerosis". Neuroepidemiology. 27 (1): 28–32. doi:10.1159/000094233. PMID 16804331. S2CID 20870484.
- Heesen C, Mohr DC, Huitinga I, Bergh FT, Gaab J, Otte C, Gold SM (March 2007). "Stress regulation in multiple sclerosis: current issues and concepts". Multiple Sclerosis. 13 (2): 143–148. doi:10.1177/1352458506070772. PMID 17439878. S2CID 8262595.
- Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S (February 2001). "Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group". The New England Journal of Medicine. 344 (5): 319–326. doi:10.1056/NEJM200102013440501. PMID 11172162.
- Martinelli V (2000). "Trauma, stress and multiple sclerosis". Neurological Sciences. 21 (4 Suppl 2): S849–S852. doi:10.1007/s100720070024. PMID 11205361. S2CID 2376078.
- Makhani N, Tremlett H (August 2021). "The multiple sclerosis prodrome". Nature Reviews. Neurology. 17 (8): 515–521. doi:10.1038/s41582-021-00519-3. PMC 8324569. PMID 34155379.
- Marrie RA (December 2019). "Mounting evidence for a multiple sclerosis prodrome". Nature Reviews. Neurology. 15 (12): 689–690. doi:10.1038/s41582-019-0283-0. PMID 31654040. S2CID 204887642.
- Kurtzke JF (October 1993). "Epidemiologic evidence for multiple sclerosis as an infection". Clinical Microbiology Reviews. 6 (4): 382–427. doi:10.1128/CMR.6.4.382. PMC 358295. PMID 8269393.
- Gilden DH (March 2005). "Infectious causes of multiple sclerosis". The Lancet. Neurology. 4 (3): 195–202. doi:10.1016/S1474-4422(05)01017-3. PMC 7129502. PMID 15721830.
- Soldan SS, Lieberman PM (January 2023). "Epstein-Barr virus and multiple sclerosis". Nature Reviews. Microbiology. 21 (1): 51–64. doi:10.1038/s41579-022-00770-5. PMC 9362539. PMID 35931816.
- Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. (January 2022). "Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis". Science. 375 (6578): 296–301. Bibcode:2022Sci...375..296B. doi:10.1126/science.abj8222. PMID 35025605. S2CID 245983763. See BBC lay summary Archived 25 April 2022 at the Wayback Machine of 13 April 2022.
- Dyment DA, Ebers GC, Sadovnick AD (February 2004). "Genetics of multiple sclerosis". The Lancet. Neurology. 3 (2): 104–10. CiteSeerX 10.1.1.334.1312. doi:10.1016/S1474-4422(03)00663-X. PMID 14747002. S2CID 16707321.
- Skene NG, Grant SG (2016). "Identification of Vulnerable Cell Types in Major Brain Disorders Using Single Cell Transcriptomes and Expression Weighted Cell Type Enrichment". Frontiers in Neuroscience. 10: 16. doi:10.3389/fnins.2016.00016. PMC 4730103. PMID 26858593.
- Hassan-Smith G, Douglas MR (October 2011). "Epidemiology and diagnosis of multiple sclerosis". British Journal of Hospital Medicine. 72 (10): M146-51. doi:10.12968/hmed.2011.72.Sup10.M146. PMID 22041658.
- Rosati G (April 2001). "The prevalence of multiple sclerosis in the world: an update". Neurological Sciences. 22 (2): 117–39. doi:10.1007/s100720170011. PMID 11603614. S2CID 207051545.
- Baranzini SE (June 2011). "Revealing the genetic basis of multiple sclerosis: are we there yet?". Current Opinion in Genetics & Development. 21 (3): 317–24. doi:10.1016/j.gde.2010.12.006. PMC 3105160. PMID 21247752.
- International Multiple Sclerosis Genetics Consortium (2019). "Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility". Science. 365 (6460). doi:10.1126/science.aav7188. PMC 7241648. PMID 31604244.
- Alonso A, Hernán MA (July 2008). "Temporal trends in the incidence of multiple sclerosis: a systematic review". Neurology. 71 (2): 129–135. doi:10.1212/01.wnl.0000316802.35974.34. PMC 4109189. PMID 18606967.
- Pugliatti M, Sotgiu S, Rosati G (July 2002). "The worldwide prevalence of multiple sclerosis". Clinical Neurology and Neurosurgery. 104 (3): 182–91. doi:10.1016/S0303-8467(02)00036-7. PMID 12127652. S2CID 862001.
- Grimaldi LM, Salemi G, Grimaldi G, Rizzo A, Marziolo R, Lo Presti C, Maimone D, Savettieri G (November 2001). "High incidence and increasing prevalence of MS in Enna (Sicily), southern Italy". Neurology. 57 (10): 1891–3. doi:10.1212/wnl.57.10.1891. PMID 11723283. S2CID 34895995.
- Kulie T, Groff A, Redmer J, Hounshell J, Schrager S (2009). "Vitamin D: an evidence-based review". Journal of the American Board of Family Medicine. 22 (6): 698–706. doi:10.3122/jabfm.2009.06.090037. PMID 19897699.
- Marrie RA (December 2004). "Environmental risk factors in multiple sclerosis aetiology". The Lancet. Neurology. 3 (12): 709–18. doi:10.1016/S1474-4422(04)00933-0. PMID 15556803. S2CID 175786.
- Ascherio A, Munger KL (June 2007). "Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors". Annals of Neurology. 61 (6): 504–13. doi:10.1002/ana.21141. PMID 17492755. S2CID 36999504.
- Hedström A, Hössjer O, Katsoulis M (September 2018). "Organic solvents and MS susceptibility: Interaction with MS risk HLA genes". Neurology. 91 (5): 455–462. doi:10.1212/WNL.0000000000005906. PMC 6093765. PMID 29970406.
- Stowe J, Andrews N, Miller E (January 2020). "Do Vaccines Trigger Neurological Diseases? Epidemiological Evaluation of Vaccination and Neurological Diseases Using Examples of Multiple Sclerosis, Guillain-Barré Syndrome and Narcolepsy". CNS Drugs. 34 (1): 1–8. doi:10.1007/s40263-019-00670-y. PMC 7224038. PMID 31576507.
- Spitsin S, Koprowski H (2008). "Role of Uric Acid in Multiple Sclerosis". Advances in multiple Sclerosis and Experimental Demyelinating Diseases. Current Topics in Microbiology and Immunology. Vol. 318. pp. 325–342. doi:10.1007/978-3-540-73677-6_13. ISBN 978-3-540-73676-9. PMID 18219824.
- Nourbakhsh B, Mowry EM (June 2019). "Multiple Sclerosis Risk Factors and Pathogenesis". Continuum. 25 (3): 596–610. doi:10.1212/CON.0000000000000725. PMID 31162307. S2CID 174806511.
- Chen Y, Popko B (2018). "Cholesterol crystals impede nerve repair". Science. 359 (6376): 635–636. Bibcode:2018Sci...359..635C. doi:10.1126/science.aar7369. PMID 29439228. S2CID 3257111.
- Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, Möbius W, Simons M (2018). "Defective cholesterol clearance limits remyelination in the aged central nervous system". Science. 359 (6376): 684–688. Bibcode:2018Sci...359..684C. doi:10.1126/science.aan4183. PMID 29301957.
- Chari DM (2007). "Remyelination in multiple sclerosis". International Review of Neurobiology. 79: 589–620. doi:10.1016/S0074-7742(07)79026-8. ISBN 978-0-12-373736-6. PMC 7112255. PMID 17531860.
- Pittock SJ, Lucchinetti CF (March 2007). "The pathology of MS: new insights and potential clinical applications". The Neurologist. 13 (2): 45–56. doi:10.1097/01.nrl.0000253065.31662.37. PMID 17351524. S2CID 2993523.
- Huang X, Hussain B, Chang J (January 2021). "Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms". CNS Neuroscience & Therapeutics. 27 (1): 36–47. doi:10.1111/cns.13569. PMC 7804893. PMID 33381913.
- Ferré JC, Shiroishi MS, Law M (November 2012). "Advanced techniques using contrast media in neuroimaging". Magnetic Resonance Imaging Clinics of North America. 20 (4): 699–713. doi:10.1016/j.mric.2012.07.007. PMC 3479680. PMID 23088946.
- Trojano M, Paolicelli D (November 2001). "The differential diagnosis of multiple sclerosis: classification and clinical features of relapsing and progressive neurological syndromes". Neurological Sciences. 22 (Suppl 2): S98-102. doi:10.1007/s100720100044. PMID 11794488. S2CID 3057096.
- World Health Organization (2008). Atlas: Multiple Sclerosis Resources in the World 2008. Geneva: World Health Organization. pp. 15–16. hdl:10665/43968. ISBN 978-92-4-156375-8.
- Poser CM, Brinar VV (June 2004). "Diagnostic criteria for multiple sclerosis: an historical review". Clinical Neurology and Neurosurgery. 106 (3): 147–58. doi:10.1016/j.clineuro.2004.02.004. PMID 15177763. S2CID 23452341.
- Rovira À (November 2017). "Diagnosis of Multiple Sclerosis". Journal of the Belgian Society of Radiology. 101 (S1): 12. doi:10.5334/jbr-btr.1426.
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (July 2001). "Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis". Annals of Neurology. 50 (1): 121–7. doi:10.1002/ana.1032. PMID 11456302. S2CID 13870943.
- Rashid W, Miller DH (February 2008). "Recent advances in neuroimaging of multiple sclerosis". Seminars in Neurology. 28 (1): 46–55. doi:10.1055/s-2007-1019127. PMID 18256986. S2CID 260317568.
- Sinnecker T, Clarke MA, Meier D, Enzinger C, Calabrese M, De Stefano N, et al. (MAGNIMS Study Group) (December 2019). "Evaluation of the Central Vein Sign as a Diagnostic Imaging Biomarker in Multiple Sclerosis". JAMA Neurology. 76 (12): 1446–1456. doi:10.1001/jamaneurol.2019.2478. PMC 6704746. PMID 31424490.
- Bernitsas E (February 2020). "The Central Vein Sign". Practical Neurology. Archived from the original on 5 October 2021. Retrieved 5 October 2021.
- Castellaro M, Tamanti A, Pisani AI, Pizzini FB, Crescenzo F, Calabrese M (November 2020). "The Use of the Central Vein Sign in the Diagnosis of Multiple Sclerosis: A Systematic Review and Meta-analysis". Diagnostics. 10 (12): 1025. doi:10.3390/diagnostics10121025. PMC 7760678. PMID 33260401.
- Al-Zandi SH, Fayadh NA, Al-Waely NK (1 March 2018). "Central vein sign detected by SWI at 3 T MRI as a discriminator between multiple sclerosis and leukoaraiosis". The Egyptian Journal of Radiology and Nuclear Medicine. 49 (1): 158–164. doi:10.1016/j.ejrnm.2017.09.003.
- Guisset F, Lolli V, Bugli C, Perrotta G, Absil J, Dachy B, et al. (June 2021). "The central vein sign in multiple sclerosis patients with vascular comorbidities" (PDF). Multiple Sclerosis. 27 (7): 1057–1065. doi:10.1177/1352458520943785. hdl:2078.1/239849. PMID 32749948. S2CID 220976821. Archived (PDF) from the original on 15 July 2021. Retrieved 5 October 2021.
- Chapman M (16 June 2020). "$7.2M NIH Grant Supports Study of MS Diagnostic Biomarker". BioNews Services. Archived from the original on 5 October 2021. Retrieved 5 October 2021.
- Andravizou A, Dardiotis E, Artemiadis A, Sokratous M, Siokas V, Tsouris Z, et al. (December 2019). "Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options". Auto- Immunity Highlights. 10 (1): 7. doi:10.1186/s13317-019-0117-5. PMC 7065319. PMID 32257063.
- Jacobsen C, Hagemeier J, Myhr KM, Nyland H, Lode K, Bergsland N, et al. (October 2014). "Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study". Journal of Neurology, Neurosurgery, and Psychiatry. 85 (10): 1109–1115. doi:10.1136/jnnp-2013-306906. PMID 24554101. S2CID 6144791.
- Link H, Huang YM (November 2006). "Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness". Journal of Neuroimmunology. 180 (1–2): 17–28. doi:10.1016/j.jneuroim.2006.07.006. PMID 16945427. S2CID 22724352.
- Loscalzo J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL (2022). Harrison's principles of internal medicine (21st ed.). New York. ISBN 978-1-264-26849-8. OCLC 1282172709.
{{cite book}}: CS1 maint: location missing publisher (link) - Saguil A, Farnell Iv EA, Jordan TS (August 2022). "Multiple Sclerosis: A Primary Care Perspective". American Family Physician. 106 (2): 173–183. PMID 35977131.
- Solomon AJ (June 2019). "Diagnosis, Differential Diagnosis, and Misdiagnosis of Multiple Sclerosis". Continuum. 25 (3): 611–635. doi:10.1212/CON.0000000000000728. PMID 31162308. S2CID 173991777.
- Lublin FD, et al. (15 July 2014). "Defining the clinical course of multiple sclerosis, The 2013 revisions". Neurology. 83 (3): 278–286. doi:10.1212/WNL.0000000000000560. PMC 4117366. PMID 24871874.
- Lublin FD, Coetzee T, Cohen JA, Marrie RA, Thompson AJ (June 2020). "The 2013 clinical course descriptors for multiple sclerosis: A clarification". Neurology. 94 (24): 1088–1092. doi:10.1212/WNL.0000000000009636. PMC 7455332. PMID 32471886.
- "Clinically Isolated Syndrome (CIS)". National MS Society. Retrieved 4 October 2023.
- Miller D, Barkhof F, Montalban X, Thompson A, Filippi M (May 2005). "Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis". The Lancet. Neurology. 4 (5): 281–8. doi:10.1016/S1474-4422(05)70071-5. PMID 15847841. S2CID 36401666.
- Miller DH, Leary SM (October 2007). "Primary-progressive multiple sclerosis". The Lancet. Neurology. 6 (10): 903–12. doi:10.1016/S1474-4422(07)70243-0. hdl:1871/24666. PMID 17884680. S2CID 31389841.
- Rovaris M, Confavreux C, Furlan R, Kappos L, Comi G, Filippi M (April 2006). "Secondary progressive multiple sclerosis: current knowledge and future challenges". The Lancet. Neurology. 5 (4): 343–54. doi:10.1016/S1474-4422(06)70410-0. PMID 16545751. S2CID 39503553.
- Sørensen PS, Centonze D, Giovannoni G, et al. (2020). "Expert opinion on the use of cladribine tablets in clinical practice". Ther Adv Neurol Disord (Review). 13: 1756286420935019. doi:10.1177/1756286420935019. PMC 7318823. PMID 32636933.
- "Novartis receives FDA approval for Mayzent® (siponimod), the first oral drug to treat secondary progressive MS with active disease". Novartis.com. Archived from the original on 20 November 2020. Retrieved 12 November 2021.
- Saida T (November 2004). "[Multiple sclerosis: treatment and prevention of relapses and progression in multiple sclerosis]". Rinsho Shinkeigaku (Review) (in Japanese). 44 (11): 796–8. PMID 15651294.
- Pittock SJ, Rodriguez M (2008). "Benign Multiple Sclerosis: A Distinct Clinical Entity with Therapeutic Implications". Advances in multiple Sclerosis and Experimental Demyelinating Diseases. Current Topics in Microbiology and Immunology. Vol. 318. pp. 1–17. doi:10.1007/978-3-540-73677-6_1. ISBN 978-3-540-73676-9. PMID 18219812.
- Feinstein A (2007). "1. Multiple sclerosis: diagnosis and definitions". The Clinical Neuropsychiatry of Multiple Sclerosis. Cambridge: Cambridge University Press. p. 20. doi:10.1017/CBO9780511543760. ISBN 978-0-521-85234-0.
- Stadelmann C, Brück W (November 2004). "Lessons from the neuropathology of atypical forms of multiple sclerosis". Neurological Sciences. 25 (Suppl 4): S319–S322. doi:10.1007/s10072-004-0333-1. PMID 15727225. S2CID 21212935.
- Fujihara K (June 2019). "Neuromyelitis optica spectrum disorders: still evolving and broadening". Current Opinion in Neurology (Review). 32 (3): 385–394. doi:10.1097/WCO.0000000000000694. PMC 6522202. PMID 30893099.
- Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BA, Gronseth GS, et al. (April 2018). "Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology". Neurology. 90 (17): 777–788. doi:10.1212/WNL.0000000000005347. PMID 29686116.
- Burton JM, O'Connor PW, Hohol M, Beyene J (December 2012). "Oral versus intravenous steroids for treatment of relapses in multiple sclerosis". The Cochrane Database of Systematic Reviews. 12: CD006921. doi:10.1002/14651858.CD006921.pub3. PMID 23235634.
- Filippini G, Brusaferri F, Sibley WA, et al. (2000). "Corticosteroids or ACTH for acute exacerbations in multiple sclerosis". Cochrane Database Syst Rev (4): CD001331. doi:10.1002/14651858.CD001331. PMID 11034713.
- The National Collaborating Centre for Chronic Conditions (2004). "Treatment of acute episodes". Multiple sclerosis : national clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians. pp. 54–58. ISBN 1-86016-182-0. PMID 21290636. Archived from the original on 10 February 2023. Retrieved 5 October 2021.
- Petzold A, Braithwaite T, van Oosten BW (January 2020). "Case for a new corticosteroid treatment trial in optic neuritis: review of updated evidence". J. Neurol. Neurosurg. Psychiatry (Review). 91 (1): 9–14. doi:10.1136/jnnp-2019-321653. PMC 6952848. PMID 31740484.
- He D, Zhang C, Zhao X, Zhang Y, Dai Q, Li Y, Chu L (March 2016). "Teriflunomide for multiple sclerosis". The Cochrane Database of Systematic Reviews. 2016 (3): CD009882. doi:10.1002/14651858.CD009882.pub3. PMC 10493042. PMID 27003123.
- Rice GP, Incorvaia B, Munari L, et al. (2001). "Interferon in relapsing-remitting multiple sclerosis". Cochrane Database Syst Rev. 2001 (4): CD002002. doi:10.1002/14651858.CD002002. PMC 7017973. PMID 11687131.
- Hassan-Smith G, Douglas MR (November 2011). "Management and prognosis of multiple sclerosis". British Journal of Hospital Medicine. 72 (11): M174-6. doi:10.12968/hmed.2011.72.Sup11.M174. PMID 22082979.
- Freedman MS (January 2011). "Long-term follow-up of clinical trials of multiple sclerosis therapies". Neurology. 76 (1 Suppl 1): S26-34. doi:10.1212/WNL.0b013e318205051d. PMID 21205679. S2CID 16929304.
- Qizilbash N, Mendez I, Sanchez-de la Rosa R (January 2012). "Benefit-risk analysis of glatiramer acetate for relapsing-remitting and clinically isolated syndrome multiple sclerosis". Clinical Therapeutics. 34 (1): 159–176.e5. doi:10.1016/j.clinthera.2011.12.006. PMID 22284996.
- Bates D (January 2011). "Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials". Neurology. 76 (1 Suppl 1): S14-25. doi:10.1212/WNL.0b013e3182050388. PMID 21205678. S2CID 362182.
- Clerico M, Faggiano F, Palace J, et al. (April 2008). "Recombinant interferon beta or glatiramer acetate for delaying conversion of the first demyelinating event to multiple sclerosis". Cochrane Database Syst Rev (2): CD005278. doi:10.1002/14651858.CD005278.pub3. PMID 18425915.
- Johnston J, So TY (June 2012). "First-line disease-modifying therapies in paediatric multiple sclerosis: a comprehensive overview". Drugs. 72 (9): 1195–211. doi:10.2165/11634010-000000000-00000. PMID 22642799. S2CID 20323687.
- La Mantia L, Tramacere I, Firwana B, et al. (April 2016). "Fingolimod for relapsing-remitting multiple sclerosis". Cochrane Database Syst Rev. 2016 (4): CD009371. doi:10.1002/14651858.CD009371.pub2. PMC 10401910. PMID 27091121.
- Xu Z, Zhang F, Sun F, et al. (April 2015). "Dimethyl fumarate for multiple sclerosis". Cochrane Database Syst Rev (4): CD011076. doi:10.1002/14651858.CD011076.pub2. PMID 25900414.
- Killestein J, Rudick RA, Polman CH (November 2011). "Oral treatment for multiple sclerosis". The Lancet. Neurology. 10 (11): 1026–34. doi:10.1016/S1474-4422(11)70228-9. PMID 22014437. S2CID 206160178.
- Filippini G, Del Giovane C, Clerico M, et al. (April 2017). "Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis". Cochrane Database Syst Rev. 2017 (4): CD012200. doi:10.1002/14651858.CD012200.pub2. PMC 6478290. PMID 28440858.
- Filippini G, Del Giovane C, Vacchi L, et al. (June 2013). "Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis" (PDF). Cochrane Database Syst Rev (6): CD008933. doi:10.1002/14651858.CD008933.pub2. PMID 23744561. Archived (PDF) from the original on 5 October 2021. Retrieved 5 October 2021.
- La Mantia L, Di Pietrantonj C, Rovaris M, et al. (November 2016). "Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis". Cochrane Database Syst Rev. 2016 (11): CD009333. doi:10.1002/14651858.CD009333.pub3. PMC 6464642. PMID 27880972.
- Tramacere I, Del Giovane C, Salanti G, D'Amico R, Filippini G (September 2015). "Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis". Cochrane Database Syst Rev. 2015 (9): CD011381. doi:10.1002/14651858.CD011381.pub2. hdl:11380/1082490. PMC 9235409. PMID 26384035.
- "TG Therapeutics Announces FDA Approval of Briumvi (ublituximab-xiiy)" (Press release). TG Therapeutics. 28 December 2022. Archived from the original on 28 December 2022. Retrieved 29 December 2022 – via GlobeNewswire.
- Bope ET, Kellerman RD (22 December 2011). Conn's Current Therapy 2012: Expert Consult – Online and Print. Elsevier Health Sciences. pp. 662–. ISBN 978-1-4557-0738-6.
- Martinelli Boneschi F, Vacchi L, Rovaris M, Capra R, Comi G (May 2013). "Mitoxantrone for multiple sclerosis". The Cochrane Database of Systematic Reviews. 5 (5): CD002127. doi:10.1002/14651858.CD002127.pub3. hdl:2434/533488. PMID 23728638.
- Marriott JJ, Miyasaki JM, Gronseth G, O'Connor PW (May 2010). "Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology". Neurology. 74 (18): 1463–70. doi:10.1212/WNL.0b013e3181dc1ae0. PMC 2871006. PMID 20439849.
- Faissner S, Gold R (2019). "Progressive multiple sclerosis: latest therapeutic developments and future directions". Therapeutic Advances in Neurological Disorders. 12: 1756286419878323. doi:10.1177/1756286419878323. PMC 6764045. PMID 31598138.
- Winslow R (28 March 2017). "After 40-year odyssey, first drug for aggressive MS wins FDA approval". STAT. Archived from the original on 1 April 2017.
- "Ocrevus- ocrelizumab injection". DailyMed. 13 December 2019. Archived from the original on 27 June 2020. Retrieved 26 March 2020.
- "BLA Approval Letter" (PDF). FDA. 28 March 2017. Archived (PDF) from the original on 2 April 2017.
- Lin M, Zhang J, Zhang Y, Luo J, Shi S, et al. (Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group) (May 2022). "Ocrelizumab for multiple sclerosis". The Cochrane Database of Systematic Reviews. 2022 (5): CD013247. doi:10.1002/14651858.CD013247.pub2. PMC 9115862. PMID 35583174.
- Penner IK, Schreiber H (2023). Fatigue in Multiple Sclerosis. Springer, Cham. ISBN 978-3-031-13498-2.
- Balak DM, Hengstman GJ, Çakmak A, Thio HB (December 2012). "Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review". Multiple Sclerosis. 18 (12): 1705–17. doi:10.1177/1352458512438239. hdl:1765/73097. PMID 22371220. S2CID 20343951. Archived from the original on 24 January 2021. Retrieved 5 September 2019.
- Sládková T, Kostolanský F (2006). "The role of cytokines in the immune response to influenza A virus infection". Acta Virologica. 50 (3): 151–62. PMID 17131933.
- La Mantia L, Munari LM, Lovati R (May 2010). "Glatiramer acetate for multiple sclerosis". The Cochrane Database of Systematic Reviews (5): CD004678. doi:10.1002/14651858.CD004678.pub2. PMID 20464733.
- Tremlett H, Oger J (November 2004). "Hepatic injury, liver monitoring and the beta-interferons for multiple sclerosis". Journal of Neurology. 251 (11): 1297–303. doi:10.1007/s00415-004-0619-5. PMID 15592724. S2CID 12529733.
- Comi G (October 2009). "Treatment of multiple sclerosis: role of natalizumab". Neurological Sciences. 30. 30 (S2): S155-8. doi:10.1007/s10072-009-0147-2. PMID 19882365. S2CID 25910077.
- Hunt D, Giovannoni G (February 2012). "Natalizumab-associated progressive multifocal leucoencephalopathy: a practical approach to risk profiling and monitoring". Practical Neurology. 12 (1): 25–35. doi:10.1136/practneurol-2011-000092. PMID 22258169. S2CID 46326042.
- "Biogen Idec's TECFIDERA™ (Dimethyl Fumarate) Approved in US as a First-Line Oral Treatment for Multiple Sclerosis" (Press release). Biogen Idec. 27 March 2013. Archived from the original on 12 May 2013. Retrieved 4 June 2013.
- "NDA 204063 – FDA Approved Labeling Text" (PDF). US Food and Drug Agency. 27 March 2013. Archived (PDF) from the original on 4 October 2013. Retrieved 5 April 2013.
"NDA Approval" (PDF). US Food and Drug Agency. 27 March 2013. Archived (PDF) from the original on 4 October 2013. Retrieved 5 April 2013. - Kesselring J, Beer S (October 2005). "Symptomatic therapy and neurorehabilitation in multiple sclerosis". The Lancet. Neurology. 4 (10): 643–52. doi:10.1016/S1474-4422(05)70193-9. PMID 16168933. S2CID 28253186.
- Khan F, Turner-Stokes L, Ng L, Kilpatrick T (April 2007). Khan F (ed.). "Multidisciplinary rehabilitation for adults with multiple sclerosis". The Cochrane Database of Systematic Reviews. 2011 (2): CD006036. doi:10.1002/14651858.CD006036.pub2. PMC 8992048. PMID 17443610.
- Köpke S, Solari A, Rahn A, Khan F, Heesen C, Giordano A (October 2018). "Information provision for people with multiple sclerosis". The Cochrane Database of Systematic Reviews. 10 (10): CD008757. doi:10.1002/14651858.CD008757.pub3. PMC 6517040. PMID 30317542.
- Steultjens EM, Dekker J, Bouter LM, Leemrijse CJ, van den Ende CH (May 2005). "Evidence of the efficacy of occupational therapy in different conditions: an overview of systematic reviews" (PDF). Clinical Rehabilitation. 19 (3): 247–54. doi:10.1191/0269215505cr870oa. hdl:1871/26505. PMID 15859525. S2CID 18785849. Archived (PDF) from the original on 4 August 2020. Retrieved 24 September 2019.
- Steultjens EM, Dekker J, Bouter LM, Cardol M, Van de Nes JC, Van den Ende CH (2003). Steultjens EE (ed.). "Occupational therapy for multiple sclerosis" (PDF). The Cochrane Database of Systematic Reviews. 2010 (3): CD003608. doi:10.1002/14651858.CD003608. PMC 9022193. PMID 12917976. Archived (PDF) from the original on 28 April 2022. Retrieved 5 October 2021.
- Amatya B, Khan F, Galea M (January 2019). "Rehabilitation for people with multiple sclerosis: an overview of Cochrane Reviews". The Cochrane Database of Systematic Reviews. 1 (1): CD012732. doi:10.1002/14651858.CD012732.pub2. PMC 6353175. PMID 30637728.
- Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G (September 2015). "Exercise therapy for fatigue in multiple sclerosis". The Cochrane Database of Systematic Reviews. 2015 (9): CD009956. doi:10.1002/14651858.CD009956.pub2. PMC 9554249. PMID 26358158.
- Gallien P, Nicolas B, Robineau S, Pétrilli S, Houedakor J, Durufle A (July 2007). "Physical training and multiple sclerosis". Annales de Réadaptation et de Médecine Physique. 50 (6): 373–6, 369–72. doi:10.1016/j.annrmp.2007.04.004. PMID 17482708.
- Rietberg MB, Brooks D, Uitdehaag BM, Kwakkel G (January 2005). Kwakkel G (ed.). "Exercise therapy for multiple sclerosis". The Cochrane Database of Systematic Reviews. 2005 (1): CD003980. doi:10.1002/14651858.CD003980.pub2. PMC 6485797. PMID 15674920.
- Thomas PW, Thomas S, Hillier C, Galvin K, Baker R (January 2006). Thomas PW (ed.). "Psychological interventions for multiple sclerosis". The Cochrane Database of Systematic Reviews. 2010 (1): CD004431. doi:10.1002/14651858.CD004431.pub2. PMC 8406851. PMID 16437487.
- Rosti-Otajärvi EM, Hämäläinen PI (February 2014). "Neuropsychological rehabilitation for multiple sclerosis". The Cochrane Database of Systematic Reviews (2): CD009131. doi:10.1002/14651858.CD009131.pub3. PMID 24515630.
- Latorraca CO, Martimbianco AL, Pachito DV, Torloni MR, Pacheco RL, Pereira JG, Riera R (October 2019). "Palliative care interventions for people with multiple sclerosis". The Cochrane Database of Systematic Reviews. 2019 (10): CD012936. doi:10.1002/14651858.CD012936.pub2. PMC 6803560. PMID 31637711.
- Hayes S, Galvin R, Kennedy C, Finlayson M, McGuigan C, Walsh CD, Coote S (November 2019). "Interventions for preventing falls in people with multiple sclerosis". The Cochrane Database of Systematic Reviews. 2019 (11): CD012475. doi:10.1002/14651858.CD012475.pub2. PMC 6953359. PMID 31778221.
- van den Akker LE, Beckerman H, Collette EH, Eijssen IC, Dekker J, de Groot V (November 2016). "Effectiveness of cognitive behavioral therapy for the treatment of fatigue in patients with multiple sclerosis: A systematic review and meta-analysis". Journal of Psychosomatic Research. 90: 33–42. doi:10.1016/j.jpsychores.2016.09.002. PMID 27772557.
- Amatya B, Young J, Khan F (December 2018). "Non-pharmacological interventions for chronic pain in multiple sclerosis". The Cochrane Database of Systematic Reviews. 12 (12): CD012622. doi:10.1002/14651858.CD012622.pub2. PMC 6516893. PMID 30567012.
- Corvillo I, Varela E, Armijo F, Alvarez-Badillo A, Armijo O, Maraver F (December 2017). "Efficacy of aquatic therapy for multiple sclerosis: a systematic review". Eur J Phys Rehabil Med (Review). 53 (6): 944–952. doi:10.23736/S1973-9087.17.04570-1. PMID 28215060.
- Khan F, Amatya B, Bensmail D, Yelnik A (July 2019). "Non-pharmacological interventions for spasticity in adults: An overview of systematic reviews". Ann Phys Rehabil Med. 62 (4): 265–273. doi:10.1016/j.rehab.2017.10.001. PMID 29042299. S2CID 207497395.
- Etoom M, Khraiwesh Y, Lena F, et al. (November 2018). "Effectiveness of Physiotherapy Interventions on Spasticity in People With Multiple Sclerosis: A Systematic Review and Meta-Analysis". Am J Phys Med Rehabil. 97 (11): 793–807. doi:10.1097/PHM.0000000000000970. PMID 29794531. S2CID 44156766.
- Olsen SA (2009). "A review of complementary and alternative medicine (CAM) by people with multiple sclerosis". Occupational Therapy International. 16 (1): 57–70. doi:10.1002/oti.266. PMID 19222053.
- Parks NE, Jackson-Tarlton CS, Vacchi L, Merdad R, Johnston BC (May 2020). "Dietary interventions for multiple sclerosis-related outcomes". The Cochrane Database of Systematic Reviews. 2020 (5): CD004192. doi:10.1002/14651858.CD004192.pub4. PMC 7388136. PMID 32428983.
- Grigorian A, Araujo L, Naidu NN, Place DJ, Choudhury B, Demetriou M (November 2011). "N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis". The Journal of Biological Chemistry. 286 (46): 40133–41. doi:10.1074/jbc.M111.277814. PMC 3220534. PMID 21965673.
- Pozuelo-Moyano B, Benito-León J, Mitchell AJ, Hernández-Gallego J (2013). "A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in multiple sclerosis". Neuroepidemiology (Systematic review). 40 (3): 147–53. doi:10.1159/000345122. PMC 3649517. PMID 23257784.
the available evidence substantiates neither clinically significant benefit nor harm from vitamin D in the treatment of patients with MS
- Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E (October 2006). "Cannabis use in patients with multiple sclerosis". Multiple Sclerosis. 12 (5): 646–51. doi:10.1177/1352458506070947. PMID 17086912. S2CID 34692470.
- Torres-Moreno MC, Papaseit E, Torrens M, Farré M (October 2018). "Assessment of Efficacy and Tolerability of Medicinal Cannabinoids in Patients With Multiple Sclerosis: A Systematic Review and Meta-analysis". JAMA Network Open. 1 (6): e183485. doi:10.1001/jamanetworkopen.2018.3485. PMC 6324456. PMID 30646241.
- Bennett M, Heard R (2004). Bennett MH (ed.). "Hyperbaric oxygen therapy for multiple sclerosis". The Cochrane Database of Systematic Reviews. 2011 (1): CD003057. doi:10.1002/14651858.CD003057.pub2. PMC 8407327. PMID 14974004.
- Adams T (23 May 2010). "Gut instinct: the miracle of the parasitic hookworm". The Observer. Archived from the original on 24 October 2014.
- Simpson R, Booth J, Lawrence M, Byrne S, Mair F, Mercer S (January 2014). "Mindfulness based interventions in multiple sclerosis—a systematic review". BMC Neurology. 14: 15. doi:10.1186/1471-2377-14-15. PMC 3900731. PMID 24438384.
- Jagannath VA, Filippini G, Di Pietrantonj C, Asokan GV, Robak EW, Whamond L, Robinson SA (September 2018). "Vitamin D for the management of multiple sclerosis". The Cochrane Database of Systematic Reviews. 9 (9): CD008422. doi:10.1002/14651858.CD008422.pub3. PMC 6513642. PMID 30246874.
- Motte J, Gold R (December 2020). "High-dose biotin in multiple sclerosis: the end of the road". Lancet Neurol. 19 (12): 965–966. doi:10.1016/S1474-4422(20)30353-7. PMID 33222766. S2CID 225049079.
- Tryfonos C, Mantzorou M, Fotiou D, Vrizas M, Vadikolias K, Pavlidou E, Giaginis C (September 2019). "Dietary Supplements on Controlling Multiple Sclerosis Symptoms and Relapses: Current Clinical Evidence and Future Perspectives". Medicines. 6 (3): 95. doi:10.3390/medicines6030095. PMC 6789617. PMID 31547410.
- Sedel F, Bernard D, Mock DM, Tourbah A (November 2016). "Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis". Neuropharmacology. 110 (Pt B): 644–653. doi:10.1016/j.neuropharm.2015.08.028. PMID 26327679.
- Goldschmidt CH, Cohen JA (July 2020). "The Rise and Fall of High-Dose Biotin to Treat Progressive Multiple Sclerosis". Neurotherapeutics. 17 (3): 968–970. doi:10.1007/s13311-020-00907-5. PMC 7609671. PMID 32761325.
- Cree BA, Hartung HP, Barnett M (June 2022). "New drugs for multiple sclerosis: new treatment algorithms". Curr Opin Neurol. 35 (3): 262–270. doi:10.1097/WCO.0000000000001063. PMID 35674067. S2CID 249438715.
- Oh J, Vidal-Jordana A, Montalban X (December 2018). "Multiple sclerosis: clinical aspects". Curr Opin Neurol. 31 (6): 752–759. doi:10.1097/WCO.0000000000000622. PMID 30300239. S2CID 6103857.
- Hauser SL, Cree BA (December 2020). "Treatment of Multiple Sclerosis: A Review". Am J Med. 133 (12): 1380–1390.e2. doi:10.1016/j.amjmed.2020.05.049. PMC 7704606. PMID 32682869.
- Ontaneda D (June 2019). "Progressive Multiple Sclerosis". Continuum (Minneap Minn). 25 (3): 736–752. doi:10.1212/CON.0000000000000727. PMID 31162314. S2CID 174808956.
- Inojosa H, Proschmann U, Akgün K, Ziemssen T (April 2021). "A focus on secondary progressive multiple sclerosis (SPMS): challenges in diagnosis and definition". J Neurol. 268 (4): 1210–1221. doi:10.1007/s00415-019-09489-5. PMID 31363847. S2CID 198999832. Expanded Disability Status Scale (EDSS) 6.0 is equivalent to requiring a walking aid, and EDSS 8.0 equivalent to chair or bedbound
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (April 2018). "Multiple sclerosis". Lancet. 391 (10130): 1622–1636. doi:10.1016/S0140-6736(18)30481-1. PMID 29576504. S2CID 4313310.
- Lunde HM, Assmus J, Myhr KM, Bø L, Grytten N (August 2017). "Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study". Journal of Neurology, Neurosurgery, and Psychiatry. 88 (8): 621–625. doi:10.1136/jnnp-2016-315238. PMC 5537547. PMID 28365589.
- "Atlas of MS" (PDF). Multiple Sclerosis International Federation. September 2020.
- Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. (December 2020). "Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition". Multiple Sclerosis. 26 (14): 1816–1821. doi:10.1177/1352458520970841. PMC 7720355. PMID 33174475.
- Compston A (October 1988). "The 150th anniversary of the first depiction of the lesions of multiple sclerosis". Journal of Neurology, Neurosurgery, and Psychiatry. 51 (10): 1249–52. doi:10.1136/jnnp.51.10.1249. PMC 1032909. PMID 3066846.
- Lassmann H (October 1999). "The pathology of multiple sclerosis and its evolution". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 354 (1390): 1635–40. doi:10.1098/rstb.1999.0508. PMC 1692680. PMID 10603616.
- Lassmann H (July 2005). "Multiple sclerosis pathology: evolution of pathogenetic concepts". Brain Pathology. 15 (3): 217–22. doi:10.1111/j.1750-3639.2005.tb00523.x. PMC 8095927. PMID 16196388. S2CID 8342303.
- Milo R, Miller A (April 2014). "Revised diagnostic criteria of multiple sclerosis". Autoimmunity Reviews. 13 (4–5): 518–524. doi:10.1016/j.autrev.2014.01.012. PMID 24424194.
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (February 2011). "Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria". Annals of Neurology. 69 (2): 292–302. doi:10.1002/ana.22366. PMC 3084507. PMID 21387374.
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O'Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stüve O, Waubant E, Polman CH (July 2014). "Defining the clinical course of multiple sclerosis: the 2013 revisions". Neurology. 83 (3): 278–86. doi:10.1212/WNL.0000000000000560. PMC 4117366. PMID 24871874.
- Sørensen PS, Centonze D, Giovannoni G, Montalban X, Selchen D, Vermersch P, et al. (24 June 2020). "Expert opinion on the use of cladribine tablets in clinical practice". Therapeutic Advances in Neurological Disorders. 13: 1756286420935019. doi:10.1177/1756286420935019. PMC 7318823. PMID 32636933.
- Medaer R (September 1979). "Does the history of multiple sclerosis go back as far as the 14th century?". Acta Neurologica Scandinavica. 60 (3): 189–92. doi:10.1111/j.1600-0447.1979.tb08970.x. PMID 390966. S2CID 221422840.
- Holmøy T (2006). "A Norse contribution to the history of neurological diseases". European Neurology. 55 (1): 57–8. doi:10.1159/000091431. PMID 16479124.
- Firth D (1948). The Case of August D'Esté. Cambridge: Cambridge University Press.
- Pearce JM (2005). "Historical descriptions of multiple sclerosis". European Neurology. 54 (1): 49–53. doi:10.1159/000087387. PMID 16103678.
- Barbellion WN (1919). The Journal of a Disappointed Man. New York: George H. Doran. ISBN 0-7012-1906-8.
- Petzold A (2013). "Optic Neuritis: Another Dickensian Diagnosis". Neuro-Ophthalmology. 37 (6): 247–250. doi:10.3109/01658107.2013.830313. PMC 5291069. PMID 28167994.
- Bar-Or A, Pender MP, Khanna R, Steinman L, Hartung HP, Maniar T, et al. (March 2020). "Epstein-Barr Virus in Multiple Sclerosis: Theory and Emerging Immunotherapies". Trends in Molecular Medicine. 26 (3): 296–310. doi:10.1016/j.molmed.2019.11.003. PMC 7106557. PMID 31862243.
- DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A (June 2006). "Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up". Archives of Neurology. 63 (6): 839–844. doi:10.1001/archneur.63.6.noc50328. PMID 16606758.
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. (January 2017). "Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis". The New England Journal of Medicine. 376 (3): 221–234. doi:10.1056/NEJMoa1601277. hdl:2445/178507. PMID 28002679. S2CID 205099904.
- Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, et al. (March 2022). "Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM". Nature. 603 (7900): 321–327. Bibcode:2022Natur.603..321L. doi:10.1038/s41586-022-04432-7. PMC 9382663. PMID 35073561.
- Morandi E, Tanasescu R, Tarlinton RE, Constantinescu CS, Zhang W, Tench C, Gran B (16 February 2017). Ruprecht K (ed.). "The association between human endogenous retroviruses and multiple sclerosis: A systematic review and meta-analysis". PLOS ONE. 12 (2): e0172415. Bibcode:2017PLoSO..1272415M. doi:10.1371/journal.pone.0172415. PMC 5313176. PMID 28207850.
- Li Y, Fan T, Cui J (March 2022). "Human endogenous retroviruses in viral disease and therapy". Clinical and Translational Discovery. 2 (1). doi:10.1002/ctd2.38. ISSN 2768-0622. S2CID 247750447.
- Rangel SC, da Silva MD, da Silva AL, Dos Santos JM, Neves LM, Pedrosa A, et al. (23 November 2022). "Human endogenous retroviruses and the inflammatory response: A vicious circle associated with health and illness". Frontiers in Immunology. 13: 1057791. doi:10.3389/fimmu.2022.1057791. PMC 9744114. PMID 36518758.
- Yang C, Hao Z, Zhang L, Zeng L, Wen J (October 2015). "Sodium channel blockers for neuroprotection in multiple sclerosis". The Cochrane Database of Systematic Reviews. 2015 (10): CD010422. doi:10.1002/14651858.CD010422.pub2. PMC 9242538. PMID 26486929.
- Misu T, Fujihara K (February 2019). "Neuromyelitis optica spectrum and myelin oligodendrocyte glycoprotein antibody‐related disseminated encephalomyelitis". Clinical and Experimental Neuroimmunology. 10 (1): 9–17. doi:10.1111/cen3.12491.
- Kira JI, Yamasaki R, Ogata H (2019). "Anti-neurofascin autoantibody and demyelination". Neurochemistry International. 130: 104360. doi:10.1016/j.neuint.2018.12.011. PMID 30582947.
- Popescu BF, Pirko I, Lucchinetti CF (August 2013). "Pathology of multiple sclerosis: where do we stand?". Continuum (Minneapolis, Minn.). 19 (4 Multiple Sclerosis): 901–21. doi:10.1212/01.CON.0000433291.23091.65. PMC 3915566. PMID 23917093.
- Mehta V, Pei W, Yang G, Li S, Swamy E, Boster A, Schmalbrock P, Pitt D (2013). "Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions". PLOS ONE. 8 (3): e57573. Bibcode:2013PLoSO...857573M. doi:10.1371/journal.pone.0057573. PMC 3597727. PMID 23516409.
- Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. (October 2018). "Neurofilaments as biomarkers in neurological disorders" (PDF). Nature Reviews. Neurology. 14 (10): 577–589. doi:10.1038/s41582-018-0058-z. PMID 30171200. S2CID 52140127. Archived (PDF) from the original on 24 January 2021. Retrieved 19 September 2020.
- Petzold A (June 2005). "Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss" (PDF). Journal of the Neurological Sciences. 233 (1–2): 183–98. doi:10.1016/j.jns.2005.03.015. PMID 15896809. S2CID 18311152. Archived (PDF) from the original on 22 September 2017. Retrieved 19 September 2020.
- Filippi M, Rocca MA, De Stefano N, Enzinger C, Fisher E, Horsfield MA, Inglese M, Pelletier D, Comi G (December 2011). "Magnetic resonance techniques in multiple sclerosis: the present and the future". Archives of Neurology. 68 (12): 1514–20. doi:10.1001/archneurol.2011.914. PMID 22159052.
- Swanberg KM, Landheer K, Pitt D, Juchem C (2019). "Quantifying the Metabolic Signature of Multiple Sclerosis by in vivo Proton Magnetic Resonance Spectroscopy: Current Challenges and Future Outlook in the Translation From Proton Signal to Diagnostic Biomarker". Frontiers in Neurology. 10: 1173. doi:10.3389/fneur.2019.01173. PMC 6876616. PMID 31803127.
- Moghadasi AN, Mirmosayyeb O, Barzegar M, Sahraian MA, Ghajarzadeh M (August 2021). "The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): a systematic review and meta-analysis". Neurol Sci. 42 (8): 3093–3099. doi:10.1007/s10072-021-05373-1. PMC 8184129. PMID 34100130.
- Prinz M (3 November 2017). "Faculty Opinions recommendation of Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice". Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature. doi:10.3410/f.731077576.793538553.
- Aranow C (August 2011). "Vitamin D and the immune system". Journal of Investigative Medicine. 59 (6): 881–886. doi:10.2310/jim.0b013e31821b8755. PMC 3166406. PMID 21527855.
- Tada T, Takemori T, Okumura K, Nonaka M, Tokuhisa T (February 1978). "Two distinct types of helper T cells involved in the secondary antibody response: independent and synergistic effects of Ia- and Ia+ helper T cells". The Journal of Experimental Medicine. 147 (2): 446–458. doi:10.1084/jem.147.2.446. PMC 2184496. PMID 415110.
- Lassmann H (March 2018). "Multiple Sclerosis Pathology". Cold Spring Harbor Perspectives in Medicine. 8 (3): a028936. doi:10.1101/cshperspect.a028936. PMC 5830904. PMID 29358320.
- "Pathogenesis of HIV-Associated Dementia and Multiple Sclerosis: Role of Microglia and Astrocytes", The Role of Glia in Neurotoxicity, CRC Press, pp. 283–298, 28 December 2004, doi:10.1201/9781420039740-21, ISBN 9780429128608
