Phenotype
In genetics, the phenotype (from Ancient Greek φαίνω (phaínō) 'to appear, show', and τύπος (túpos) 'mark, type') is the set of observable characteristics or traits of an organism.[1][2] The term covers the organism's morphology (physical form and structure), its developmental processes, its biochemical and physiological properties, its behavior, and the products of behavior. An organism's phenotype results from two basic factors: the expression of an organism's genetic code (its genotype) and the influence of environmental factors. Both factors may interact, further affecting the phenotype. When two or more clearly different phenotypes exist in the same population of a species, the species is called polymorphic. A well-documented example of polymorphism is Labrador Retriever coloring; while the coat color depends on many genes, it is clearly seen in the environment as yellow, black, and brown. Richard Dawkins in 1978[3] and then again in his 1982 book The Extended Phenotype suggested that one can regard bird nests and other built structures such as caddisfly larva cases and beaver dams as "extended phenotypes".


Wilhelm Johannsen proposed the genotype–phenotype distinction in 1911 to make clear the difference between an organism's hereditary material and what that hereditary material produces.[4][5] The distinction resembles that proposed by August Weismann (1834–1914), who distinguished between germ plasm (heredity) and somatic cells (the body). More recently, in The Selfish Gene (1976), Dawkins distinguished these concepts as replicators and vehicles.
Definition
Despite its seemingly straightforward definition, the concept of the phenotype has hidden subtleties. It may seem that anything dependent on the genotype is a phenotype, including molecules such as RNA and proteins. Most molecules and structures coded by the genetic material are not visible in the appearance of an organism, yet they are observable (for example by Western blotting) and are thus part of the phenotype; human blood groups are an example. It may seem that this goes beyond the original intentions of the concept with its focus on the (living) organism in itself. Either way, the term phenotype includes inherent traits or characteristics that are observable or traits that can be made visible by some technical procedure.
The term "phenotype" has sometimes been incorrectly used as a shorthand for the phenotypic difference between a mutant and its wild type, which would lead to the false statement that a "mutation has no phenotype".[6]
Behaviors and their consequences are also phenotypes, since behaviors are observable characteristics. Behavioral phenotypes include cognitive, personality, and behavioral patterns. Some behavioral phenotypes may characterize psychiatric disorders[7] or syndromes.[8][9]
A phenome is the set of all traits expressed by a cell, tissue, organ, organism, or species. The term was first used by Davis in 1949, "We here propose the name phenome for the sum total of extragenic, non-autoreproductive portions of the cell, whether cytoplasmic or nuclear. The phenome would be the material basis of the phenotype, just as the genome is the material basis of the genotype."[10]
Although phenome has been in use for many years, the distinction between the use of phenome and phenotype is problematic. A proposed definition for both terms as the "physical totality of all traits of an organism or of one of its subsystems" was put forth by Mahner and Kary in 1997, who argue that although scientists tend to intuitively use these and related terms in a manner that does not impede research, the terms are not well defined and usage of the terms is not consistent.[11]
Some usages of the term suggest that the phenome of a given organism is best understood as a kind of matrix of data representing physical manifestation of phenotype. For example, discussions led by A.Varki among those who had used the term up to 2003 suggested the following definition: “The body of information describing an organism's phenotypes, under the influences of genetic and environmental factors”.[12] Another team of researchers characterize "the human phenome [as] a multidimensional search space with several neurobiological levels, spanning the proteome, cellular systems (e.g., signaling pathways), neural systems and cognitive and behavioural phenotypes."[13]
Plant biologists have started to explore the phenome in the study of plant physiology.[14]
In 2009, a research team demonstrated the feasibility of identifying genotype–phenotype associations using electronic health records (EHRs) linked to DNA biobanks. They called this method phenome-wide association study (PheWAS).[15]
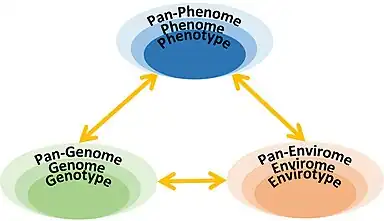
Inspired by the evolution from genotype to genome to pan-genome, a concept of exploring the relationship ultimately among pan-phenome, pan-genome, and pan-envirome was proposed in 2023.[16]


Phenotypic variation
Phenotypic variation (due to underlying heritable genetic variation) is a fundamental prerequisite for evolution by natural selection. It is the living organism as a whole that contributes (or not) to the next generation, so natural selection affects the genetic structure of a population indirectly via the contribution of phenotypes. Without phenotypic variation, there would be no evolution by natural selection.[17]
The interaction between genotype and phenotype has often been conceptualized by the following relationship:
- genotype (G) + environment (E) → phenotype (P)
A more nuanced version of the relationship is:
- genotype (G) + environment (E) + genotype & environment interactions (GE) → phenotype (P)
Genotypes often have much flexibility in the modification and expression of phenotypes; in many organisms these phenotypes are very different under varying environmental conditions. The plant Hieracium umbellatum is found growing in two different habitats in Sweden. One habitat is rocky, sea-side cliffs, where the plants are bushy with broad leaves and expanded inflorescences; the other is among sand dunes where the plants grow prostrate with narrow leaves and compact inflorescences. These habitats alternate along the coast of Sweden and the habitat that the seeds of Hieracium umbellatum land in, determine the phenotype that grows.[18]
An example of random variation in Drosophila flies is the number of ommatidia, which may vary (randomly) between left and right eyes in a single individual as much as they do between different genotypes overall, or between clones raised in different environments.
The concept of phenotype can be extended to variations below the level of the gene that affect an organism's fitness. For example, silent mutations that do not change the corresponding amino acid sequence of a gene may change the frequency of guanine-cytosine base pairs (GC content). These base pairs have a higher thermal stability (melting point) than adenine-thymine, a property that might convey, among organisms living in high-temperature environments, a selective advantage on variants enriched in GC content.
The extended phenotype
Richard Dawkins described a phenotype that included all effects that a gene has on its surroundings, including other organisms, as an extended phenotype, arguing that "An animal's behavior tends to maximize the survival of the genes 'for' that behavior, whether or not those genes happen to be in the body of the particular animal performing it."[3] For instance, an organism such as a beaver modifies its environment by building a beaver dam; this can be considered an expression of its genes, just as its incisor teeth are—which it uses to modify its environment. Similarly, when a bird feeds a brood parasite such as a cuckoo, it is unwittingly extending its phenotype; and when genes in an orchid affect orchid bee behavior to increase pollination, or when genes in a peacock affect the copulatory decisions of peahens, again, the phenotype is being extended. Genes are, in Dawkins's view, selected by their phenotypic effects.[19]
Other biologists broadly agree that the extended phenotype concept is relevant, but consider that its role is largely explanatory, rather than assisting in the design of experimental tests.[20]
Genes and phenotypes
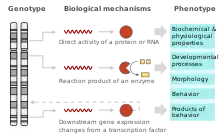
Phenotypes are determined by an interaction of genes and the environment, but the mechanism for each gene and phenotype is different. For instance, an albino phenotype may be caused by a mutation in the gene encoding tyrosinase which is a key enzyme in melanin formation. However, exposure to UV radiation can increase melanin production, hence the environment plays a role in this phenotype as well. For most complex phenotypes the precise genetic mechanism remains unknown. For instance, it is largely unclear how genes determine the shape of bones or the human ear.
Gene expression plays a crucial role in determining the phenotypes of organisms. The level of gene expression can affect the phenotype of an organism. For example, if a gene that codes for a particular enzyme is expressed at high levels, the organism may produce more of that enzyme and exhibit a particular trait as a result. On the other hand, if the gene is expressed at low levels, the organism may produce less of the enzyme and exhibit a different trait.[22]
Gene expression is regulated at various levels and thus each level can affect certain phenotypes, including transcriptional and post-transcriptional regulation.

Changes in the levels of gene expression can be influenced by a variety of factors, such as environmental conditions, genetic variations, and epigenetic modifications. These modifications can be influenced by environmental factors such as diet, stress, and exposure to toxins, and can have a significant impact on an individual's phenotype. Some phenotypes may be the result of changes in gene expression due to these factors, rather than changes in genotype. An experiment involving machine learning methods utilizing gene expression measured from RNA sequencing can contain enough signal to separate individuals in the context of phenotype prediction.[23]
Phenome and phenomics
Although a phenotype is the ensemble of observable characteristics displayed by an organism, the word phenome is sometimes used to refer to a collection of traits, while the simultaneous study of such a collection is referred to as phenomics.[24][25] Phenomics is an important field of study because it can be used to figure out which genomic variants affect phenotypes which then can be used to explain things like health, disease, and evolutionary fitness.[26] Phenomics forms a large part of the Human Genome Project.[27]
Phenomics has applications in agriculture. For instance, genomic variations such as drought and heat resistance can be identified through phenomics to create more durable GMOs.[28][29]
Phenomics may be a stepping stone towards personalized medicine, particularly drug therapy.[30] Once the phenomic database has acquired more data, a person's phenomic information can be used to select specific drugs tailored to an individual.[30]
Large-scale phenotyping and genetic screens
Large-scale genetic screens can identify the genes or mutations that affect the phenotype of an organism. Analyzing the phenotypes of mutant genes can also aid in determining gene function.[31] Most genetic screens have used microorganisms, in which genes can be easily deleted. For instance, nearly all genes have been deleted in E. coli[32] and many other bacteria, but also in several eukaryotic model organisms such as baker's yeast[33] and fission yeast.[34] Among other discoveries, such studies have revealed lists of essential genes .
More recently, large-scale phenotypic screens have also been used in animals, e.g. to study lesser understood phenotypes such as behavior. In one screen, the role of mutations in mice were studied in areas such as learning and memory, circadian rhythmicity, vision, responses to stress and response to psychostimulants.
| Phenotypic Domain | Assay | Notes | Software Package |
|---|---|---|---|
| Circadian Rhythm | Wheel running behavior | ClockLab | |
| Learning and Memory | Fear conditioning | Video-image-based scoring of freezing | FreezeFrame |
| Preliminary Assessment | Open field activity and elevated plus maze | Video-image-based scoring of exploration | LimeLight |
| Psychostimulant response | Hyperlocomotion behavior | Video-image-based tracking of locomotion | BigBrother |
| Vision | Electroretinogram and Fundus photography | L. Pinto and colleagues |
This experiment involved the progeny of mice treated with ENU, or N-ethyl-N-nitrosourea, which is a potent mutagen that causes point mutations. The mice were phenotypically screened for alterations in the different behavioral domains in order to find the number of putative mutants (see table for details). Putative mutants are then tested for heritability in order to help determine the inheritance pattern as well as map out the mutations. Once they have been mapped out, cloned, and identified, it can be determined whether a mutation represents a new gene or not.
| Phenotypic domain | ENU Progeny screened | Putative mutants | Putative mutant lines with progeny | Confirmed mutants |
|---|---|---|---|---|
| General assessment | 29860 | 80 | 38 | 14 |
| Learning and memory | 23123 | 165 | 106 | 19 |
| Psychostimulant response | 20997 | 168 | 86 | 9 |
| Neuroendocrine response to stress | 13118 | 126 | 54 | 2 |
| Vision | 15582 | 108 | 60 | 6 |
These experiments showed that mutations in the rhodopsin gene affected vision and can even cause retinal degeneration in mice.[35] The same amino acid change causes human familial blindness, showing how phenotyping in animals can inform medical diagnostics and possibly therapy.
Evolutionary origin of phenotype
The RNA world is the hypothesized pre-cellular stage in the evolutionary history of life on earth, in which self-replicating RNA molecules proliferated prior to the evolution of DNA and proteins.[36] The folded three-dimensional physical structure of the first RNA molecule that possessed ribozyme activity promoting replication while avoiding destruction would have been the first phenotype, and the nucleotide sequence of the first self-replicating RNA molecule would have been the original genotype.[36]
See also
References
- "Phenotype adjective – Definition, pictures, pronunciation and usage notes". Oxford Advanced Learner's Dictionary at OxfordLearnersDictionaries.com. Retrieved 2020-04-29.
the set of observable characteristics of an individual resulting from the interaction of its genotype with the environment.
- "Genotype versus phenotype". Understanding Evolution. Retrieved 2020-04-29.
An organism's genotype is the set of genes that it carries. An organism's phenotype is all of its observable characteristics — which are influenced both by its genotype and by the environment.
- Dawkins R (May 1978). "Replicator selection and the extended phenotype". Zeitschrift für Tierpsychologie. 47 (1): 61–76. doi:10.1111/j.1439-0310.1978.tb01823.x. PMID 696023.
- Churchill FB (1974). "William Johannsen and the genotype concept". Journal of the History of Biology. 7 (1): 5–30. doi:10.1007/BF00179291. PMID 11610096. S2CID 38649212.
- Johannsen W (August 2014). "The genotype conception of heredity. 1911". International Journal of Epidemiology. 43 (4): 989–1000. doi:10.1086/279202. JSTOR 2455747. PMC 4258772. PMID 24691957.
- Crusio WE (May 2002). "'My mouse has no phenotype'". Genes, Brain and Behavior. 1 (2): 71. doi:10.1034/j.1601-183X.2002.10201.x. PMID 12884976. S2CID 35382304.
- Cassidy SB, Morris CA (2002-01-01). "Behavioral phenotypes in genetic syndromes: genetic clues to human behavior". Advances in Pediatrics. 49: 59–86. PMID 12214780.
- O'Brien G, Yule W, eds. (1995). Behavioural Phenotype. Clinics in Developmental Medicine No.138. London: Mac Keith Press. ISBN 978-1-898683-06-3.
- O'Brien G, ed. (2002). Behavioural Phenotypes in Clinical Practice. London: Mac Keith Press. ISBN 978-1-898683-27-8. Retrieved 27 September 2010.
- Davis BD (January 1949). "The Isolation of Biochemically Deficient Mutants of Bacteria by Means of Penicillin". Proceedings of the National Academy of Sciences of the United States of America. 35 (1): 1–10. Bibcode:1949PNAS...35....1D. doi:10.1073/pnas.35.1.1. PMC 1062948. PMID 16588845.
- Loeffler M, Bratke T, Paulus U, Li YQ, Potten CS (May 1997). "Clonality and life cycles of intestinal crypts explained by a state dependent stochastic model of epithelial stem cell organization". Journal of Theoretical Biology. 186 (1): 41–54. Bibcode:1997JThBi.186...41L. doi:10.1006/jtbi.1996.0340. PMID 9176636.
- Varki A, Altheide TK (December 2005). "Comparing the human and chimpanzee genomes: searching for needles in a haystack". Genome Research. 15 (12): 1746–58. doi:10.1101/gr.3737405. PMID 16339373.
- Siebner HR, Callicott JH, Sommer T, Mattay VS (November 2009). "From the genome to the phenome and back: linking genes with human brain function and structure using genetically informed neuroimaging". Neuroscience. 164 (1): 1–6. doi:10.1016/j.neuroscience.2009.09.009. PMC 3013363. PMID 19751805.
- Furbank, Robert T.; Tester, Mark (December 2011). "Phenomics--technologies to relieve the phenotyping bottleneck". Trends in Plant Science. 16 (12): 635–644. doi:10.1016/j.tplants.2011.09.005. ISSN 1878-4372. PMID 22074787.
- Denny, Joshua C.; Ritchie, Marylyn D.; Basford, Melissa A.; Pulley, Jill M.; Bastarache, Lisa; Brown-Gentry, Kristin; Wang, Deede; Masys, Dan R.; Roden, Dan M.; Crawford, Dana C. (2010-05-01). "PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations". Bioinformatics. 26 (9): 1205–1210. doi:10.1093/bioinformatics/btq126. ISSN 1367-4811. PMC 2859132. PMID 20335276.
- Guo, Tingting; Li, Xianran (2023). "Machine learning for predicting phenotype from genotype and environment". Current Opinion in Biotechnology. 79: 102853. doi:10.1016/j.copbio.2022.102853. PMID 36463837. S2CID 254211407.
- Lewontin RC (November 1970). "The Units of Selection" (PDF). Annual Review of Ecology and Systematics. 1: 1–18. doi:10.1146/annurev.es.01.110170.000245. JSTOR 2096764. S2CID 84684420.
- von Sengbusch P. "Phenotypic and Genetic Variation; Ecotypes". Botany online: Evolution: The Modern Synthesis - Phenotypic and Genetic Variation; Ecotypes. Archived from the original on 2009-06-18. Retrieved 2009-12-29.
- Dawkins R (1982). The Extended Phenotype. Oxford University. p. 4. ISBN 978-0-19-288051-2.
- Hunter P (March 2009). "Extended phenotype redux. How far can the reach of genes extend in manipulating the environment of an organism?". EMBO Reports. 10 (3): 212–215. doi:10.1038/embor.2009.18. PMC 2658563. PMID 19255576.
- Pakay, Julian; Duivenvoorden, Hendrika; Shafee, Thomas; Clarke, Kaitlin (2023). Threshold Concepts in Biochemistry. La Trobe eBureau. doi:10.26826/1017. ISBN 978-0-6484681-9-6. S2CID 258899183.
- Oellrich, A.; Sanger Mouse Genetics Project; Smedley, D. (2014). "Linking tissues to phenotypes using gene expression profiles". Database. 2014: bau017. doi:10.1093/database/bau017. PMC 3982582. PMID 24634472.
- Nussinov, Ruth; Tsai, Chung-Jung; Jang, Hyunbum (2019). "Protein ensembles link genotype to phenotype". PLOS Computational Biology. 15 (6): e1006648. Bibcode:2019PLSCB..15E6648N. doi:10.1371/journal.pcbi.1006648. PMC 6586255. PMID 31220071.
- Mahner M, Kary M (May 1997). "What exactly are genomes, genotypes and phenotypes? And what about phenomes?". Journal of Theoretical Biology. 186 (1): 55–63. Bibcode:1997JThBi.186...55M. doi:10.1006/jtbi.1996.0335. PMID 9176637.
- Varki A, Wills C, Perlmutter D, Woodruff D, Gage F, Moore J, et al. (October 1998). "Great Ape Phenome Project?". Science. 282 (5387): 239–240. Bibcode:1998Sci...282..239V. doi:10.1126/science.282.5387.239d. PMID 9841385. S2CID 5837659.
- Houle D, Govindaraju DR, Omholt S (December 2010). "Phenomics: the next challenge". Nature Reviews Genetics. 11 (12): 855–866. doi:10.1038/nrg2897. PMID 21085204. S2CID 14752610.
- Freimer N, Sabatti C (May 2003). "The human phenome project". Nature Genetics. 34 (1): 15–21. doi:10.1038/ng0503-15. PMID 12721547. S2CID 31510391.
- Rahman H, Ramanathan V, Jagadeeshselvam N, Ramasamy S, Rajendran S, Ramachandran M, et al. (2015-01-01). "Phenomics: Technologies and Applications in Plant and Agriculture". In Barh D, Khan MS, Davies E (eds.). PlantOmics: The Omics of Plant Science. New Delhi: Springer. pp. 385–411. doi:10.1007/978-81-322-2172-2_13. ISBN 9788132221715.
- Furbank RT, Tester M (December 2011). "Phenomics – technologies to relieve the phenotyping bottleneck". Trends in Plant Science. 16 (12): 635–644. doi:10.1016/j.tplants.2011.09.005. PMID 22074787.
- Monte AA, Brocker C, Nebert DW, Gonzalez FJ, Thompson DC, Vasiliou V (September 2014). "Improved drug therapy: triangulating phenomics with genomics and metabolomics". Human Genomics. 8 (1): 16. doi:10.1186/s40246-014-0016-9. PMC 4445687. PMID 25181945.
- Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, et al. (October 1999). "A large-scale insertional mutagenesis screen in zebrafish". Genes & Development. 13 (20): 2713–2724. doi:10.1101/gad.13.20.2713. PMC 317115. PMID 10541557.
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. (January 2006). "Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection". Molecular Systems Biology. 2 (1): 2006.0008. doi:10.1038/msb4100050. PMC 1681482. PMID 16738554.
- Nislow C, Wong LH, Lee AH, Giaever G (September 2016). "Functional genomics using the Saccharomyces cerevisiae yeast deletion collections". Cold Spring Harbor Protocols. 2016 (9): pdb.top080945. doi:10.1101/pdb.top080945. PMID 27587784.
- Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, et al. (June 2010). "Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe". Nature Biotechnology. 28 (6): 617–623. doi:10.1038/nbt.1628. PMC 3962850. PMID 20473289.
- Vitaterna MH, Pinto LH, Takahashi JS (April 2006). "Large-scale mutagenesis and phenotypic screens for the nervous system and behavior in mice". Trends in Neurosciences. 29 (4): 233–240. doi:10.1016/j.tins.2006.02.006. PMC 3761413. PMID 16519954.
- Michod RE (February 1983). "Population biology of the first replicators: on the origin of the genotype, phenotype and organism". American Zoologist. 23 (1): 5–14. doi:10.1093/icb/23.1.5.