Myxozoa
Myxozoa (etymology: Greek: μύξα myxa "slime" or "mucus"[1] + thematic vowel o + ζῷον zoon "animal"[2]) is a subphylum of aquatic cnidarian animals – all obligate parasites. It contains the smallest animals ever known to have lived. Over 2,180 species have been described and some estimates have suggested at least 30,000 undiscovered species.[3] Many have a two-host lifecycle, involving a fish and an annelid worm or a bryozoan. The average size of a myxosporean spore usually ranges from 10 μm to 20 μm,[4] whereas that of a malacosporean (a subclade of the Myxozoa) spore can be up to 2 mm. Myxozoans can live in both freshwater and marine habitats.
| Myxozoa | |
|---|---|
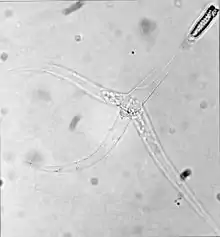 | |
| Triactinomyxon stage of Myxobolus cerebralis | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Cnidaria |
| Subphylum: | Myxozoa Grassé, 1970 |
| Classes | |
Myxozoans are highly derived cnidarians that have undergone dramatic evolution from a free swimming, self-sufficient jellyfish-like creature into their current form of obligate parasites composed of very few cells – sometimes only a single cell . As myxozoans evolved into microscopic parasites, they lost many genes responsible for multicellular development, coordination, cell–cell communication, and even, in some cases, aerobic respiration. The genomes of some myxozoans are now among the smallest genomes of any known animal species.[5][6]
Life cycle and pathology
Myxozoans are endoparasitic animals exhibiting complex life cycles that, in most of the documented cases, involve an intermediate host, usually a fish, but in rare cases amphibians,[7] reptiles,[7] birds,[8] and mammals;[9][10] and a definitive host, usually an annelid or an ectoproct.

Only about 100 life cycles have been resolved and it is suspected that there may be some exclusively terrestrial.[11] The mechanism of infection occurs through valve spores that have many forms, but their main morphology is the same: one or two sporoplasts, which are the real infectious agent, surrounded by a layer of attenuated cells called valve cells, which can secrete a layer protective coating and form float appendages. Integrated into the layer of valve cells are two to four specialized capsulogenic cells (in a few cases, one or even 15), each carrying a polar capsule containing coiled polar filaments, an extrudable organelle used for recognition, contact and infiltration.[12] Myxospores are ingested by annelids, in which the polar filaments extrude to anchor the spore to the gut epithelium. Opening of the shell valves allows the sporoplasms to penetrate into the epithelium. Subsequently, the parasite undergoes reproduction and development in the gut tissue, and finally produces usually eight actinosporean spore stages (actinospores) within a pansporocyst. After mature actinospores are released from their hosts they float in the water column.[13] Upon contact with skin or gills of fish, sporoplasms penetrate through the epithelium, followed by development of the myxosporean stage. Myxosporean trophozoites are characterized by cell-in-cell state, where the secondary (daughter) cells develop in the mother (primary) cells. The presporogonic stages multiply, migrate via nervous or circulatory systems, and develop into sporogonic stages. At the final site of infection, they produce mature spores within mono- or di-sporic pseudoplasmodia, or poly-sporic plasmodia.[14]
Relationships between myxosporeans and their hosts are often highly evolved and do not usually result in severe diseases of the natural host. Infection in fish hosts can be extremely long-lasting, potentially persisting for the lifetime of the host. However, an increasing number of myxosporeans have become commercially important pathogens of fish, largely as a result of aquaculture bringing new species into contact with myxosporeans to which they had not been previously exposed, and to which they are highly susceptible. The economic impact of such parasites can be severe, especially where prevalence rates are high; they may also have a severe impact on wild fish stocks.
The economically most significant diseases worldwide caused by myxosporeas in cultured fishes are proliferative kidney disease (PKD) caused by the malacosporean T. bryosalmonae, and whirling disease, caused by a myxosporean M. cerebralis; both diseases affect salmon. Enteromyxosis is caused by E. leei in cultured marine sparids, while proliferative gill disease (or “hamburger disease”) is caused by H. ictaluri in catfish and S. renicola infections occur in common carp.
Anatomy
Myxozoans are very small animals, typically 10–300 μm in length.[15]
Like other cnidarians they possess cnidocysts, which were referred to as "polar capsules" before the discovery that myxozoans are cnidarians. These cnidocysts fire tubules as in other cnidarians; some inject substances into the host. However, the tubules lack hooks or barbs, and in some species are more elastic than in other cnidarians.
Myxozoans have secondarily lost epithelial structures, a nervous system, gut, and cilia. Most lack muscles, though these are retained in some members of malacosporea. Those who have lost their muscles move around inside the host using other forms of locomotion, such as the use of filopodia, spore valve contractions, amoeboid movements, and rapidly creating and reabsorbing folds on the cell membrane.[16] Myxozoans do not undergo embryogenesis during development and have lost true gametes.[3] Instead, they reproduce via multicellular spores. These spores contain the polar capsules, which are not typically present in somatic cells. Centrioles are not involved in the nuclear division of myxozoans. Cell division by binary fission is rare, and cells divide instead via endogeny.[15]
In 2020, the myxozoan Henneguya salminicola was found to lack a mitochondrial genome, and thus be incapable of aerobic respiration; it was the first animal to be positively identified as such. Its actual metabolism is currently unknown.[17]
Phylogenetics
Myxozoans were originally considered to be protozoans,[18] and were included among other non-motile forms in the group Sporozoa.[19] As their distinct nature became clear through 18S ribosomal DNA (rDNA) sequencing, they were relocated in the metazoa. Detailed classification within the metazoa was however long hindered by conflicting rDNA evidence: although 18S rDNA suggested an affinity with Cnidaria,[20] other rDNA sampled,[21][22] and the HOX genes of two species,[23] were more similar to those of the Bilateria.
The discovery that Buddenbrockia plumatellae, a worm-like parasite of bryozoans up to 2 mm in length, is a myxozoan[21] initially appeared to strengthen the case for a bilaterian origin, as the body plan is superficially similar. Nevertheless, closer examination reveals that Buddenbrockia's longitudinal symmetry is not twofold, but fourfold, casting doubt on this hypothesis.
Further testing resolved the genetic conundrum by sourcing the first three previously identified discrepant HOX genes (Myx1-3) to the bryozoan Cristatella mucedo and the fourth (Myx4) to northern pike, the respective hosts of the two corresponding Myxozoa samples.[24] This explained the confusion: the original experiments had used samples contaminated by tissue from host organisms, leading to false positives for a position among the Bilateria. More careful cloning of 50 coding genes from Buddenbrockia firmly established the clade as severely modified members of the phylum Cnidaria, with medusozoans as their closest relatives.[24] Similarities between myxozoan polar capsules and cnidarian nematocysts had been drawn for a long time, but were generally assumed to be the result of convergent evolution.
Taxonomists now recognize the outdated subgroup Actinosporea as a life-cycle phase of Myxosporea.[25]
Molecular clocks suggest that myxozoans and their closest relatives, the polypodiozoa, shared their last common ancestor with medusazoans about 600 million years ago, during the Ediacaran period.[3]
Taxonomy
Myxozoan taxonomy has undergone great and important changes in its levels of generic, family and suborder classification. Fiala et al. (2015) proposed a new classification based on spores.[26]
| Phylum: Cnidaria | |
| Subphylum: Myxozoa[26] | |
| Class: Malacosporea | Class: Myxosporea (sequel) |
| Order: Malacovalvulida | Order: Bivalvulida (sequel) |
| Family: Saccosporidae | Family: Myxobilatidae |
| Genus: Buddenbrockia, Tetracapsuloides | Genus: Myxobilatus, Acauda, Hoferellus |
| Class: Myxosporea | Family: Chloromyxidae |
| Order: Bivalvulida | Genus: Chloromyxum, Caudomyxum, Agarella |
| Suborder: Variisporina | Family: Coccomyxidae |
| Family: Sphaeromyxidae | Genus: Coccomyxa, Auerbachia, Globospora |
| Genus: Sphaeromyxa | Family: Alatosporidae |
| Family: Myxidiidae | Genus: Alatospora, Pseudalatospora, Renispora |
| Genus: Myxidium, Zschokkella, Enteromyxum, Sigmomyxa, Soricimyxum, Cystodiscus | Family: Parvicapsulidae |
| Family: Ortholineidae | Genus: Parvicapsula, Neoparvicapsula, Gadimyxa |
| Genus: Ortholinea, Neomyxobolus, Cardimyxobolus, Triangula, Kentmoseria | Suborder: Platysporina |
| Family: Sinuolineidae | Family: Myxobolidae |
| Genus: Sinuolinea, Myxodavisia, Myxoproteus, Bipteria, Paramyxoproteus, Neobipteria, Schulmania, Noblea, Latyspora | Genus: Myxobolus, Spirosuturia, Unicauda, Dicauda, Phlogospora, Laterocaudata, Henneguya, Hennegoides, Tetrauronema, Thelohanellus, Neothelohanellus, Neohenneguya, Trigonosporus |
| Family: Fabesporidae | Order: Multivalvulida |
| Genus: Fabespora | Family: Trilosporidae |
| Family: Ceratomyxidae | Genus: Trilospora, Unicapsula |
| Genus: Ceratomyxa, Meglitschia, Ellipsomyxa, Ceratonova | Family: Kudoidae |
| Family: Sphaerosporidae | Genus: Kudoa |
| Genus: Sphaerospora, Wardia, Palliatus | Family: Spinavaculidae |
| Genus: Octospina | |
| Incertae sedis in Multivalvulida: Trilosporoides | |
Phylum: Cnidaria |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
References
- μύξα. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
- ζῷον. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
- Atkinson, Stephen D.; Bartholomew, Jerri L.; Lotan, Tamar (1 August 2018). "Myxozoans: Ancient metazoan parasites find a home in phylum Cnidaria". Zoology. 129: 66–68. doi:10.1016/j.zool.2018.06.005. ISSN 0944-2006. PMID 30170750. S2CID 52141614.
- Fiala, Ivan (10 July 2008). "Myxozoa". tolweb.org (under construction). The Tree of Life Web Project.
- Chang, E. Sally; Neuhof, Moran; Rubinstein, Nimrod D.; Diamant, Arik; Philippe, Hervé; Huchon, Dorothée; Cartwright, Paulyn (1 December 2015). "Genomic insights into the evolutionary origin of Myxozoa within Cnidaria". Proceedings of the National Academy of Sciences. 112 (48): 14912–14917. Bibcode:2015PNAS..11214912C. doi:10.1073/pnas.1511468112. ISSN 1091-6490. PMC 4672818. PMID 26627241.
- Yahalomi, D.; Atkinson, S.D.; Neuhof, M.; Chang, E.S.; Philippe, H.; Cartwright, P.; Bartholomew, J.L.; Huchon, D. (24 February 2020). "A cnidarian parasite of salmon (Myxozoa: Henneguya) lacks a mitochondrial genome". Proceedings of the National Academy of Sciences. 117 (10): 5358–5363. Bibcode:2020PNAS..117.5358Y. doi:10.1073/pnas.1909907117. PMC 7071853. PMID 32094163.
- Eiras, Jorge C. (2005). "An overview on the myxosporean parasites in amphibians and reptiles" (PDF). Acta Parasitologica. 50 (4): 267–275. ISSN 1230-2821.
- Bartholomew, J.L.; Atkinson S.D.; Hallett, S.L.; Lowenstine, L.J.; Garner, M.M.; Gardiner, C.H.; Rideout, B.A.; Keel, M.K.; Brown, J.D. (2008). "Myxozoan parasitism in waterfowl". International Journal for Parasitology. 38 (10): 1199–1207. doi:10.1016/j.ijpara.2008.01.008. PMID 18342316.
- Prunescu, Carol-Constantin; Prunescu, Paula; Lom, Jiří (2007). "The first finding of myxosporean development from plasmodia to spores in terrestrial mammals: Soricimyxum fegati gen. et sp. n. (Myxozoa) from Sorex araneus (Soricomorpha)" (PDF). Folia Parasitologica. 54 (3): 159–164. doi:10.14411/fp.2007.022. PMID 19245186. S2CID 45278079.
- Székely, Csaba; Cech,Gábor; Atkinson, Stephen D.; Kálmán Molnár; Egyed, László; Gubányi, András (2015). "A novel myxozoan parasite of terrestrial mammals: Description of Soricimyxum minuti sp. n. (Myxosporea) in pygmy shrew Sorex minutus from Hungary" (PDF). Folia Parasitologica. 62 (1): 45–49. doi:10.14411/fp.2015.045. PMID 26370293.
- Hallett, Sascha L.; Bartholomew, Jerri L.; Atkinson, Stephen D.; Székely, Csaba (2015). "Myxozoans exploiting homeotherms". In Okamura, B.; Gruhl, A.; Bartholomew, J.L. (eds.). Myxozoan Evolution, Ecology, and Development. Springer International Publishing. pp. 125–138. doi:10.1007/978-3-319-14753-6_7. ISBN 978-3-319-14752-9. S2CID 83229156.
- Gruhl, Alexander (2015). "Chapter 7 - Myxozoa". In Wanninger, Andreas (ed.). Evolutionary developmental biology of invertebrates. Vol. 1: Introduction, non–bilateria, acoelomorpha, xenoturbellida, chaetognatha. Springer Verlag Wien. pp. 165–177. doi:10.1007/978-3-7091-1862-7_7. ISBN 978-3-7091-1861-0.
- el Matbouli, M.; Hoffmann, R.W. (1998). "Light and electron microscopic studies on the chronological development of Myxobolus cerebralis to the actinosporean stage in Tubifex tubifex". International Journal for Parasitology. 28 (1): 195–217. doi:10.1016/s0020-7519(97)00176-8. PMID 9504346.
- el Matbouli, M.; Hoffmann, R.W.; Mandok, C. (1995). "Light and electron microscopic observations on the route of the triactinomyxon-sporoplasm of Myxobolus cerebralis from epidermis into rainbow trout (Oncorhynchus mykiss) cartilage". Journal of Fish Biology. 46 (6): 919–935. doi:10.1111/j.1095-8649.1995.tb01397.x.
- Canning, Elizabeth U.; Okamura, Beth (1 January 2003). "Biodiversity and Evolution of the Myxozoa". Advances in Parasitology. Vol. 56. Academic Press. pp. 43–131. doi:10.1016/S0065-308X(03)56002-X. ISBN 978-0-12-031756-1. PMID 14710996.
- Hartigan, A.; Estensoro, I.; Vancová, M.; Bílý, T.; Patra, S.; Eszterbauer, E.; Holzer, A. S. (16 December 2016). "New cell motility model observed in parasitic cnidarian Sphaerospora molnari (Myxozoa:Myxosporea) blood stages in fish". Scientific Reports. 6 (1): 39093. Bibcode:2016NatSR...639093H. doi:10.1038/srep39093. PMC 5159882. PMID 27982057.
- Yahalomi, Dayana; Atkinson, Stephen D.; Neuhof, Moran; Chang, E. Sally; Philippe, Hervé; Cartwright, Paulyn; Bartholomew, Jerri L.; Huchon, Dorothée (10 March 2020). "A cnidarian parasite of salmon (Myxozoa: Henneguya) lacks a mitochondrial genome". Proceedings of the National Academy of Sciences. 117 (10): 5358–5363. Bibcode:2020PNAS..117.5358Y. doi:10.1073/pnas.1909907117. ISSN 0027-8424. PMC 7071853. PMID 32094163.
- Štolc, A. (1899). "Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies". Bull. Int. l'Acad. Sci. Bohème. 12: 1–12.
- Edwin Lanfranco, 2007, A phylogenetic classification of organisms other than animals.
- Smothers, J.F.; et al. (September 1994). "Molecular evidence that the myxozoan protists are metazoans". Science. 265 (5179): 1719–1721. Bibcode:1994Sci...265.1719S. doi:10.1126/science.8085160. PMID 8085160.
- A.S. Monteiro; et al. (1 June 2002). "Orphan worm finds a home: Buddenbrockia is a Myxozoan". Mol. Biol. Evol. 19 (6): 968–71. doi:10.1093/oxfordjournals.molbev.a004155. PMID 12032254.
- J. Zrzavy & V. Hypsa (April 2003). "Myxozoa, Polypodium, and the origin of the Bilateria: The phylogenetic position of "Endocnidozoa" in light of the rediscovery of Buddenbrockia". Cladistics. 19 (2): 164–169. Bibcode:2002clad.book.....S. doi:10.1111/j.1096-0031.2003.tb00305.x. S2CID 221583517.
- C. L. Anderson, E. U. Canning & B. Okamura (March 1999). "A triploblast origin for Myxozoa?". Nature. 392 (6674): 346–347. Bibcode:1998Natur.392..346A. doi:10.1038/32801. PMID 9537319. S2CID 4426181.
- E. Jímenez-Guri; et al. (July 2007). "Buddenbrockia is a cnidarian worm". Science. 317 (116): 116–118. Bibcode:2007Sci...317..116J. doi:10.1126/science.1142024. PMID 17615357. S2CID 5170702.
- Kent M. L.; Margolis L.; Corliss J.O. (1994). "The demise of a class of protists: taxonomic and nomenclatural revisions proposed for the protist phylum Myxozoa Grasse, 1970". Canadian Journal of Zoology. 72 (5): 932–937. doi:10.1139/z94-126.
- Fiala, Ivan; Bartošová-Sojková, Pavla; Whipps, Christopher M. (2015). "Classification and Phylogenetics of Myxozoa". In Okamura, Beth; Gruhl, Alexander; Bartholomew, Jerri L. (eds.). Myxozoan Evolution, Ecology, and Development. Springer International Publishing. pp. 85–110. doi:10.1007/978-3-319-14753-6_5. ISBN 978-3-319-14752-9.
External links
- "Myxozoa". Tree of Life.
- "Myxozoan researchers network" (main page).