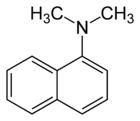
N,N-Dimethyl-1-naphthylamine
N,N-Dimethyl-1-naphthylamine is an aromatic amine. It is formally derived from 1-naphthylamine by replacing the hydrogen atoms on the amino group with methyl groups. N,N-Dimethyl-1-naphthylamine is used in the nitrate reductase test to form a red precipitate of Prontosil by reacting with a nitrite-sulfanilic acid complex.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylnaphthalen-1-amine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.530 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1][2] | |
| C12H13N | |
| Molar mass | 171.243 g·mol−1 |
| Density | 1.042 g/cm3 at 25 °C |
| Boiling point | 139 to 140 °C (282 to 284 °F; 412 to 413 K) at 13 mmHg |
| Related compounds | |
Related compounds |
1-naphthylamine 1-naphthol naphthalene aniline dimethylaniline Proton Sponge |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- "N,N-dimethylnaphthalen-1-amine". ChemSpider. Retrieved 15 April 2010.
- "D4011 N,N-Dimethyl-1-naphthylamine, ≥98.0% (GC)". Sigma-Aldrich. Retrieved 15 April 2010.
- "73426 (Fluka) Nitrate Reduction Test". Sigma-Aldrich. Retrieved 15 April 2010.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.