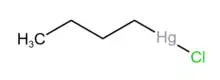
n-Butylmercuric chloride
n-Butylmercuric chloride is an organic mercury salt that is used as a catalyst and a precursor to other oganomercuric compounds.[1]
 | |
| Names | |
|---|---|
| IUPAC name
butyl(chloro)mercury | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | BMC |
| ChemSpider | |
| ECHA InfoCard | 100.190.233 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2810 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H9ClHg | |
| Molar mass | 293.16 g·mol−1 |
| Appearance | Liquid |
| Boiling point | 130 °C (266 °F; 403 K) |
| log P | 2.4411 |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H300, H310, H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P302+P352, P304+P340, P320, P321, P330, P361+P364, P391, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
73 mg/kg (rat subcutaneous) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
n-Butylmercuric chloride is made by reacting n-butylmagnesium bromide with mercury chloride.[1]

It can also be prepared by reacting 1-butene with mercury acetate.[2]
References
- Ito, Hiroshi; Trinque, Brian; Kasai, Paul (January 22, 2008). "Penultimate effect in radical copolymerization of 2-trifluoromethylacrylates". Journal of Polymer Science Part A: Polymer Chemistry. 46 (5): 1559–1565. Bibcode:2008JPoSA..46.1559I. doi:10.1002/pola.22467 – via Wiley Online Library.
- Larock, Richard C.; Brown, Herbert Charles (1970-04-01). "Organoboranes. X. Fast reaction of organoboranes with mercuric acetate. Convenient procedure for the conversion of terminal olefins into alkylmercuric salts via hydroboration-mercuration". Journal of the American Chemical Society. 92 (8): 2467–2471. doi:10.1021/ja00711a043. ISSN 0002-7863.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.