N-Oxalylglycine
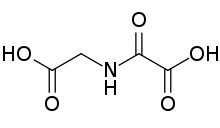
N-Oxalylglycine is the organic compound with the formula HO2CC(O)NHCH2CO2H. This colourless solid is used as an inhibitor of α-ketoglutarate-dependent enzymes.[1] It is isosteric with α-Ketoglutaric acid. Such enzymes are pervasive and, for example, are required for the synthesis of 4-hydroxyproline.
 | |
| Names | |
|---|---|
| IUPAC name
N-Oxaloglycine | |
| Systematic IUPAC name
[(Carboxymethyl)amino](oxo)acetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | NOG |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.213.188 |
| MeSH | oxalylglycine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H5NO5 | |
| Molar mass | 147.086 g·mol−1 |
| Appearance | Colorless solid |
| log P | 1.232 |
| Acidity (pKa) | 2.827 |
| Basicity (pKb) | 11.170 |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Hausinger, R. P."Fe(II)/α-ketoglutarate-dependent hydroxylases and related enzymes" Critical Reviews of Biochemical Molecular Biology 2004, 39: pp 21-68. doi:10.1080/10409230490440541
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.