N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical.
| |||
| Names | |||
|---|---|---|---|
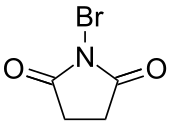
| Preferred IUPAC name
1-Bromopyrrolidine-2,5-dione | |||
| Other names
N-bromosuccinimide; NBS | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 113916 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.435 | ||
| EC Number |
| ||
| 26634 | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H4BrNO2 | |||
| Molar mass | 177.985 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 2.098 g/cm3 (solid) | ||
| Melting point | 175 to 178 °C (347 to 352 °F; 448 to 451 K) | ||
| Boiling point | 339 °C (642 °F; 612 K) | ||
| 14.7 g/L (25 °C) | |||
| Solubility in CCl4 | Insoluble (25 °C) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Irritant | ||
| Safety data sheet (SDS) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Preparation
NBS is commercially available. It can also be synthesized in the laboratory. To do so, sodium hydroxide and bromine are added to an ice-water solution of succinimide. The NBS product precipitates and can be collected by filtration.[1]
Crude NBS gives better yield in the Wohl-Ziegler reaction. In other cases, impure NBS (slightly yellow in color) may give unreliable results. It can be purified by recrystallization from 90 to 95 °C water (10 g of NBS for 100 mL of water).[2]
Reactions
Addition to alkenes
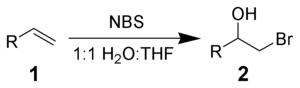
NBS will react with alkenes 1 in aqueous solvents to give bromohydrins 2. The preferred conditions are the portionwise addition of NBS to a solution of the alkene in 50% aqueous DMSO, DME, THF, or tert-butanol at 0 °C.[3] Formation of a bromonium ion and immediate attack by water gives strong Markovnikov addition and anti stereochemical selectivities.[4]
Side reactions include the formation of α-bromoketones and dibromo compounds. These can be minimized by the use of freshly recrystallized NBS.
With the addition of nucleophiles, instead of water, various bifunctional alkanes can be synthesized.[5]
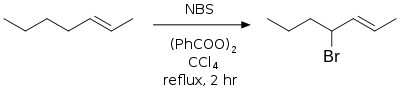
Allylic and benzylic bromination
Standard conditions for using NBS in allylic and/or benzylic bromination involves refluxing a solution of NBS in anhydrous CCl4 with a radical initiator—usually azobisisobutyronitrile (AIBN) or benzoyl peroxide, irradiation, or both to effect radical initiation.[6][7] The allylic and benzylic radical intermediates formed during this reaction are more stable than other carbon radicals and the major products are allylic and benzylic bromides. This is also called the Wohl–Ziegler reaction.[8][9]
The carbon tetrachloride must be maintained anhydrous throughout the reaction, as the presence of water may likely hydrolyze the desired product.[10] Barium carbonate is often added to maintain anhydrous and acid-free conditions.
In the above reaction, while a mixture of isomeric allylic bromide products are possible, only one is created due to the greater stability of the 4-position radical over the methyl-centered radical.
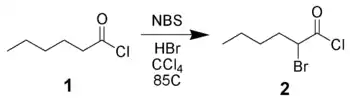
Bromination of carbonyl derivatives
NBS can α-brominate carbonyl derivatives via either a radical pathway (as above) or via acid-catalysis. For example, hexanoyl chloride 1 can be brominated in the alpha-position by NBS using acid catalysis.[11]
The reaction of enolates, enol ethers, or enol acetates with NBS is the preferred method of α-bromination as it is high-yielding with few side-products.[12][13]
Bromination of aromatic derivatives
Electron-rich aromatic compounds, such as phenols, anilines, and various aromatic heterocycles,[14] can be brominated using NBS.[15][16] Using DMF as the solvent gives high levels of para-selectivity.[17]
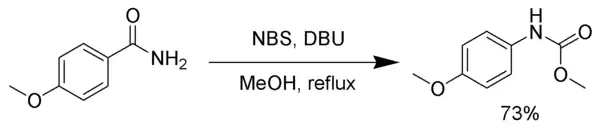
Hofmann rearrangement
NBS, in the presence of a strong base, such as DBU, reacts with primary amides to produce a carbamate via the Hofmann rearrangement.[18]
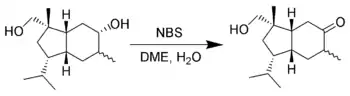
Selective oxidation of alcohols
It is uncommon, but possible for NBS to oxidize alcohols. E. J. Corey et al. found that one can selectively oxidize secondary alcohols in the presence of primary alcohols using NBS in aqueous dimethoxyethane (DME).[19]
Precautions
Although NBS is easier and safer to handle than bromine, precautions should be taken to avoid inhalation. NBS should be stored in a refrigerator. NBS will decompose over time giving off bromine. Pure NBS is white, but it is often found to be off-white or brown colored by bromine.
In general, reactions involving NBS are exothermic. Therefore, extra precautions should be taken when using on a large scale.
References
- Ziegler, K.; Späth, A. (1942). "Die Halogenierung ungesättigter Substanzen in der Allylstellungs". Ann. Chem. 551 (1): 80–119. doi:10.1002/jlac.19425510103.
- Dauben, H. J., Jr; McCoy, L. L. (1959). "N-Bromosuccinimide. I. Allylic Bromination, a General Survey of Reaction Variables". J. Am. Chem. Soc. 81 (18): 4863–4873. doi:10.1021/ja01527a027.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hanzlik, R. P. "Selective epoxidation of terminal double bonds". Organic Syntheses.; Collective Volume, vol. 6, p. 560
- Beger, J. (1991). "Präparative Aspekte elektrophiler Dreikomponentenreaktionen mit Alkenen" [Preparative aspects of electrophilic three-component reactions with alkenes]. J. Prakt. Chem. 333 (5): 677–698. doi:10.1002/prac.19913330502.
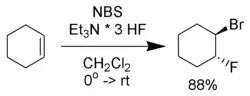
- Haufe, G.; Alvernhe, G.; Laurent, A.; Ernet, T.; Goj, O.; Kröger, S.; Sattler, A. (2004). "Bromofluorination of alkenes". Organic Syntheses.; Collective Volume, vol. 10, p. 128
- Djerassi, Carl (1948). "Brominations with N-Bromosuccinimide and Related Compounds. The Wohl–Ziegler Reaction". Chem. Rev. 43 (2): 271–317. doi:10.1021/cr60135a004. PMID 18887958.
- Greenwood, F. L.; Kellert, M. D.; Sedlak, J. (1958). "4-Bromo-2-heptene". Organic Syntheses. 38: 8. doi:10.15227/orgsyn.038.0008.
- Wohl, A. (1919). "Bromierung ungesättigter Verbindungen mit N-Brom-acetamid, ein Beitrag zur Lehre vom Verlauf chemischer Vorgänge" [Bromination of unsaturated compounds with N-bromoacetamide, a contribution to the theory of the course of chemical processes]. Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 52: 51–63. doi:10.1002/cber.19190520109.
- Ziegler, K.; Schenck, G.; Krockow, E. W.; Siebert, A.; Wenz, A.; Weber, H. (1942). "Die Synthese des Cantharidins" [The synthesis of cantharidin]. Justus Liebig's Annalen der Chemie. 551: 1–79. doi:10.1002/jlac.19425510102.
- Binkley, R. W.; Goewey, G. S.; Johnston, J. (1984). "Regioselective ring opening of selected benzylidene acetals. A photochemically initiated reaction for partial deprotection of carbohydrates". J. Org. Chem. 49 (6): 992. doi:10.1021/jo00180a008.
- Harpp, D. N.; Bao, L. Q.; Coyle, C.; Gleason, J. G.; Horovitch, S. (1988). "2-Bromohexanoyl chloride". Organic Syntheses.; Collective Volume, vol. 6, p. 190
- Stotter, P. L.; Hill, K. A. (1973). "α-Halocarbonyl compounds. II. Position-specific preparation of α-bromoketones by bromination of lithium enolates. Position-specific introduction of α,β-unsaturation into unsymmetrical ketones". J. Org. Chem. 38 (14): 2576. doi:10.1021/jo00954a045.
- Lichtenthaler, F. W. (1992). "Various Glycosyl Donors with a Ketone or Oxime Function next to the Anomeric Centre: Facile Preparation and Evaluation of their Selectivities in Glycosidations". Synthesis. 1992: 179–84. doi:10.1055/s-1992-34167.
- Amat, M.; Hadida, S.; Sathyanarayana, S.; Bosc, J. (1998). "Regioselective synthesis of 3-substituted indoles". Organic Syntheses.; Collective Volume, vol. 9, p. 417
- Gilow, H. W.; Burton, D. E. (1981). "Bromination and chlorination of pyrrole and some reactive 1-substituted pyrroles". J. Org. Chem. 46 (11): 2221. doi:10.1021/jo00324a005.
- Brown, W. D.; Gouliaev, A. H. (2005). "Synthesis of 5-bromoisoquinoline and 5-bromo-8-nitroisoquinoline". Organic Syntheses. 81: 98.
- Mitchell, R. H.; Lai, Y. H.; Williams, R. V. (1979). "N-Bromosuccinimide-dimethylformamide: a mild, selective nuclear monobromination reagent for reactive aromatic compounds". J. Org. Chem. 44 (25): 4733. doi:10.1021/jo00393a066.
- Keillor, J. W.; Huang, X. (2004). "Methyl carbamate formation via modified Hofmann rearrangement reactions". Organic Syntheses.; Collective Volume, vol. 10, p. 549
- Corey, E. J.; Ishiguro, M (1979). "Total synthesis of (±)-2-isocyanopupukeanane". Tetrahedron Lett. 20 (30): 2745–2748. doi:10.1016/S0040-4039(01)86404-2.
- Ramachandran, M. S.; Easwaramoorthy, D.; Rajasingh, V.; Vivekanandam, T. S. (1990-01-01). "N-Chlorosuccinimide-Promoted Oxidative Decarboxylation of α-Amino Acids in Aqueous Alkaline Medium". Bulletin of the Chemical Society of Japan. 63 (8): 2397–2403. doi:10.1246/bcsj.63.2397.
- Song, Xuezheng; Ju, Hong; Zhao, Chunmei; Lasanajak, Yi (2014-10-15). "Novel Strategy to Release and Tag N-Glycans for Functional Glycomics". Bioconjugate Chemistry. 25 (10): 1881–1887. doi:10.1021/bc500366v. ISSN 1043-1802. PMC 4197647. PMID 25222505.