Phenylhydroxylamine
Phenylhydroxylamine is the organic compound with the formula C6H5NHOH. It is an intermediate in the redox-related pair C6H5NH2 and C6H5NO. Phenylhydroxylamine should not be confused with its isomer α-phenylhydroxylamine or O-phenylhydroxylamine.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
N-Hydroxyaniline | |||
| Other names
beta-phenylhydroxylamine; phenylhydroxylamine; N-hydroxybenzeneamine; hydroxylaminobenzene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.614 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H7NO | |||
| Molar mass | 109.1274 g/mol | ||
| Appearance | yellow needles | ||
| Melting point | 80 to 81 °C (176 to 178 °F; 353 to 354 K) | ||
| -68.2·10−6 cm3/mol | |||
| Related compounds | |||
Related compounds |
hydroxylamine, nitrosobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Preparation
This compound can be prepared by the reduction of nitrobenzene with zinc in the presence of NH4Cl.[1][2]
Alternatively, it can be prepared by transfer hydrogenation of nitrobenzene using hydrazine as an H2 source over a rhodium catalyst.[3]
Reactions
Phenylhydroxylamine is unstable to heating, and in the presence of strong acids easily rearranges to 4-aminophenol via the Bamberger rearrangement. Oxidation of phenylhydroxylamine with dichromate gives nitrosobenzene.
The compound condenses with benzaldehyde to form diphenylnitrone, a well-known 1,3-dipole:[4] C6H5NHOH + C6H5CHO → C6H5N(O)=CHC6H5 + H2O
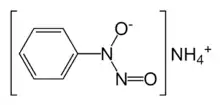
Phenylhydroxylamine is attacked by NO+ sources to give cupferron:[5]
- C6H5NHOH + C4H9ONO + NH3 → NH4[C6H5N(O)NO] + C4H9OH
References
- E. Bamberger “Ueber das Phenylhydroxylamin” Chemische Berichte, volume 27 1548-1557 (1894). E. Bamberger, "Ueber die Reduction der Nitroverbindungen" Chemische Berichte, volume 27 1347-1350 (1894) (first report)
- O. Kamm (1941). "Phenylhydroxylamine". Organic Syntheses. 4: 57. doi:10.15227/orgsyn.004.0057.
- P. W. Oxley, B. M. Adger, M. J. Sasse, M. A. Forth (1989). "N-Acetyl-N-Phenylhydroxylamine via Catalytic Transfer Hydrogenation of Nitrobenzene using Hydrazine and Rhodium on Carbon". Organic Syntheses. 67: 187. doi:10.15227/orgsyn.067.0187.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - I. Brüning, R. Grashey, H. Hauck, R. Huisgen, H. Seidl (1966). "2,3,5-Triphenylisoxazolidine". Organic Syntheses. 46: 127. doi:10.15227/orgsyn.046.0127.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - C. S. Marvel (1925). "Cupferron". Organic Syntheses. 4: 19. doi:10.15227/orgsyn.004.0019.