Non-aqueous phase liquid
Non-aqueous phase liquids, or NAPLs, are organic liquid contaminants characterized by their relative immiscibility with water. The most common examples of NAPLs include petroleum products, coal tars, halogenated solvents, wood preserving wastes, and pesticides[1]. NAPLs present dangers and challenges upon entering groundwater, which is an important source for consumption in domestic, industrial, and agricultural sectors; a relatively small volume of NAPL can create toxic groundwater conditions[2]. As such, scientists must take care to understand how NAPLs travel through subsurface systems and how they can be effectively remediated.
NAPLs can be released into the environment from a variety of point sources, e.g. improper chemical disposal and leaking underground storage tanks beneath gasoline stations. The composition of the subsurface environment influences their subsequent movement. The subsurface can be categorized into two primary zones: the unsaturated (vadose) zone and the saturated (phreatic) zone, where important storages of groundwater called aquifers are contained. Upon entering the saturated zone, NAPLs behave differently depending on their density relative to that of water. Thus, NAPLs are typically divided into two primary types: light non-aqueous phase liquids (LNAPLs) and dense non-aqueous phase liquids (DNAPLs)[2].
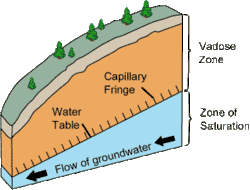
Depending on their densities, LNAPLs will either float on the water table upon reaching the saturated zone or pool at the bottom[1]. As such, the mitigation strategies differ depending on the behavior of the NAPLs; mitigation of LNAPLs tends to be less complex and require simpler engineering strategies. Conversely, DNAPLs can seep into cracks in the parent material of the subsurface, complicating both their movement and the technology required for their mitigation[2]. In terms of their measurement, traditional methods, which involve well drilling, tend to be invasive to the subsurface environment. Certain techniques that utilize NAPLs' chemical properties, such as time domain reflectometry which utilizes NAPLs' relative electrical permittivity, are in development to provide alternatives to these traditional techniques[3].
References
- "Document Display | NEPIS | US EPA". nepis.epa.gov. Retrieved 2023-10-28.
- Hemond, Harold F.; Fechner, Elizabeth J. (2023-01-01), Hemond, Harold F.; Fechner, Elizabeth J. (eds.), "Chapter 3 - The Subsurface Environment", Chemical Fate and Transport in the Environment (Fourth Edition), Boston: Academic Press, pp. 223–316, ISBN 978-0-12-822252-2, retrieved 2023-10-28
- Comegna, Alessandro; Severino, Gerardo; Coppola, Antonio (2022-10-01). "A review of new TDR applications for measuring non-aqueous phase liquids (NAPLs) in soils". Environmental Advances. 9: 100296. doi:10.1016/j.envadv.2022.100296. ISSN 2666-7657.