Nanodisc
A nanodisc is a synthetic model membrane system which assists in the study of membrane proteins.[1] Nanodiscs are discoidal proteins in which a lipid bilayer is surrounded by molecules that are amphipathic molecules including proteins, peptides, and synthetic polymers.[2] It is composed of a lipid bilayer of phospholipids with the hydrophobic edge screened by two amphipathic proteins. These proteins are called membrane scaffolding proteins (MSP) and align in double belt formation.[3][4][5] Nanodiscs are structurally very similar to discoidal high-density lipoproteins (HDL) and the MSPs are modified versions of apolipoprotein A1 (apoA1), the main constituent in HDL. Nanodiscs are useful in the study of membrane proteins because they can solubilise and stabilise membrane proteins[6] and represent a more native environment than liposomes, detergent micelles, bicelles and amphipols.
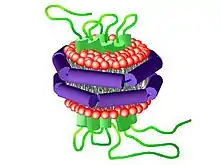
The art of making nanodiscs has progressed past using only the MSPs and lipids to make particles, leading to alternative strategies like peptide nanodiscs that use simpler proteins and synthetic nanodiscs that do not need any proteins for stabilization.
MSP nanodisc
The original nanodisc was produced by apoA1-derived MSPs from 2002.[3] The size and stability of these discs depend on the size of these proteins, which can be adjusted by truncation and fusion. In general, MSP1 proteins consist of one repeat, and MSP2s are double-sized.[7][8]
Peptide nanodisc
In peptide nanodiscs, the lipid bilayer is screened by amphipathic peptides instead of two MSPs. Peptide nanodiscs are structurally similar to MSP nanodiscs and the peptides also align in a double belt. They can stabilise membrane proteins,[9] but have higher polydispersity and are structurally less stable than MSP nanodiscs. Recent studies, however, showed that dimerization[10] and polymerization[11] of the peptides make them more stable.
Synthetic/Native nanodisc
Another way to mimic the native lipid membrane are synthetic polymers. Styrene-maleic acid co-polymers (SMAs)[12][13] called SMALPs or Lipodisq and Diisobutylene-maleic acid (DIBMA)[14] are such synthetic polymers (DIBMALPs). They can solubilize membrane proteins directly from cells or raw extract. They also have been used to study the lipid composition of several organisms.[15][16][17] It was discovered that all synthetic polymers which contained a styrene and maleic acid group can solubilize proteins.[18] These SMA nanoparticles have also been tested as possible drug delivery vehicle[19] and for the study of folding, post-translational modifications and lipid interactions of membrane proteins by native mass spectrometry.[20]
References
- Liszewski K (1 October 2015). "Dissecting the Structure of Membrane Proteins". Genetic Engineering & Biotechnology News. 35 (17): 16–18, 21. doi:10.1089/gen.35.07.09.
Nanodiscs are self-assembling nanoscale phospholipid bilayers that are stabilized using engineered membrane scaffold proteins.
- Anada, Chiharu; Ikeda, Keisuke; Egawa, Ayako; Fujiwara, Toshimichi; Nakao, Hiroyuki; Nakano, Minoru (April 2021). "Temperature- and composition-dependent conformational transitions of amphipathic peptide–phospholipid nanodiscs". Journal of Colloid and Interface Science. 588: 522–530. doi:10.1016/j.jcis.2020.12.090. ISSN 0021-9797. PMID 33429348.
- Bayburt TH, Grinkova YV, Sligar SG (2002). "Self-Assembly of Discoidal Phospholipid Bilayer Nanoparticles with Membrane Scaffold Proteins". Nano Letters. 2 (8): 853–856. Bibcode:2002NanoL...2..853B. doi:10.1021/nl025623k.
- Bayburt TH, Sligar SG (May 2010). "Membrane protein assembly into Nanodiscs". FEBS Letters. 584 (9): 1721–7. doi:10.1016/j.febslet.2009.10.024. PMC 4758813. PMID 19836392.
- Skar-Gislinge N, Simonsen JB, Mortensen K, Feidenhans'l R, Sligar SG, Lindberg Møller B, et al. (October 2010). "Elliptical structure of phospholipid bilayer nanodiscs encapsulated by scaffold proteins: casting the roles of the lipids and the protein". Journal of the American Chemical Society. 132 (39): 13713–22. doi:10.1021/ja1030613. PMC 4120756. PMID 20828154.
- Denisov IG, Sligar SG (January 2011). "Cytochromes P450 in nanodiscs". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1814 (1): 223–9. doi:10.1016/j.bbapap.2010.05.017. PMC 2974961. PMID 20685623.
- Denisov IG, Grinkova YV, Lazarides AA, Sligar SG (March 2004). "Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size". Journal of the American Chemical Society. 126 (11): 3477–87. doi:10.1021/ja0393574. PMID 15025475.
- Grinkova YV, Denisov IG, Sligar SG (November 2010). "Engineering extended membrane scaffold proteins for self-assembly of soluble nanoscale lipid bilayers". Protein Engineering, Design & Selection. 23 (11): 843–8. doi:10.1093/protein/gzq060. PMC 2953958. PMID 20817758.
- Midtgaard SR, Pedersen MC, Kirkensgaard JJ, Sørensen KK, Mortensen K, Jensen KJ, Arleth L (February 2014). "Self-assembling peptides form nanodiscs that stabilize membrane proteins". Soft Matter. 10 (5): 738–52. doi:10.1039/c3sm51727f. PMID 24651399.
- Larsen AN, Sørensen KK, Johansen NT, Martel A, Kirkensgaard JJ, Jensen KJ, et al. (July 2016). "Dimeric peptides with three different linkers self-assemble with phospholipids to form peptide nanodiscs that stabilize membrane proteins". Soft Matter. 12 (27): 5937–49. Bibcode:2016SMat...12.5937L. doi:10.1039/c6sm00495d. PMID 27306692.
- Kondo H, Ikeda K, Nakano M (October 2016). "Formation of size-controlled, denaturation-resistant lipid nanodiscs by an amphiphilic self-polymerizing peptide". Colloids and Surfaces. B, Biointerfaces. 146: 423–30. doi:10.1016/j.colsurfb.2016.06.040. PMID 27393815.
- Bada Juarez JF, Harper AJ, Judge PJ, Tonge SR, Watts A (July 2019). "From polymer chemistry to structural biology: The development of SMA and related amphipathic polymers for membrane protein extraction and solubilisation". Chemistry and Physics of Lipids. 221: 167–175. doi:10.1016/j.chemphyslip.2019.03.008. PMID 30940445.
- Knowles TJ, Finka R, Smith C, Lin YP, Dafforn T, Overduin M (June 2009). "Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer". Journal of the American Chemical Society. 131 (22): 7484–5. doi:10.1021/ja810046q. PMID 19449872.
- Oluwole AO, Klingler J, Danielczak B, Babalola JO, Vargas C, Pabst G, Keller S (December 2017). "Formation of Lipid-Bilayer Nanodiscs by Diisobutylene/Maleic Acid (DIBMA) Copolymer". Langmuir. 33 (50): 14378–14388. doi:10.1021/acs.langmuir.7b03742. PMID 29160078.
- Lavington S, Watts A (November 2020). "Lipid nanoparticle technologies for the study of G protein-coupled receptors in lipid environments". Biophysical Reviews. 12 (6): 1287–1302. doi:10.1007/s12551-020-00775-5. PMC 7755959. PMID 33215301.
- Barniol-Xicota M, Verhelst SH (February 2021). "Lipidomic and in-gel analysis of maleic acid co-polymer nanodiscs reveals differences in composition of solubilized membranes". Communications Biology. 4 (1): 218. doi:10.1038/s42003-021-01711-3. PMC 7886889. PMID 33594255.
- Bada Juarez JF, O'Rourke D, Judge PJ, Liu LC, Hodgkin J, Watts A (August 2019). "Lipodisqs for eukaryote lipidomics with retention of viability: Sensitivity and resistance to Leucobacter infection linked to C.elegans cuticle composition". Chemistry and Physics of Lipids. 222: 51–58. doi:10.1016/j.chemphyslip.2019.02.005. PMID 31102583.
- "Diisobutylene-maleic acid (DIBMA)". Cube Biotech. Retrieved 2019-02-21.
- Torgersen ML, Judge PJ, Bada Juarez JF, Pandya AD, Fusser M, Davies CW, et al. (April 2020). "Physicochemical Characterization, Toxicity and In Vivo Biodistribution Studies of a Discoidal, Lipid-Based Drug Delivery Vehicle: Lipodisq Nanoparticles Containing Doxorubicin". Journal of Biomedical Nanotechnology. 16 (4): 419–431. doi:10.1166/jbn.2020.2911. hdl:10852/85267. PMID 32970975.
- Hoi KK, Bada Juarez JF, Judge PJ, Yen HY, Wu D, Vinals J, et al. (March 2021). "Detergent-free Lipodisq Nanoparticles Facilitate High-Resolution Mass Spectrometry of Folded Integral Membrane Proteins". Nano Letters. 21 (7): 2824–2831. doi:10.1021/acs.nanolett.0c04911. PMC 8050825. PMID 33787280.
External links
- Nanodisc Technology from the Stephen Sligar laboratory
- Assembled nanodiscs for application with cell-free lysates
- HDL and Nanodiscs an overview of nanodisc technology at UIUC
- Phospholipid Bilayer Nanodiscs A summary from the Atkins lab at the University of Washington
- Purchase the MSP The plasmid for the MSP is available from AddGene
- SMA native nanodiscs website International research community website using SMA or other polymers (DIBMA for e.g.) as an alternative to conventional detergents and synthetic lipid environment found in MSP-Nanodisc.