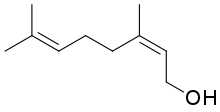
Nerol
Nerol is a monoterpenoid alcohol found in many essential oils such as lemongrass and hops. It was originally isolated from neroli oil, hence its name. This colourless liquid is used in perfumery. Like geraniol, nerol has a sweet rose odor but it is considered to be fresher.[1] Esters and related derivatives of nerol are referred to as neryl, e.g., neryl acetate.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2Z)-3,7-Dimethylocta-2,6-dien-1-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.072 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.25 g/mol |
| Density | 0.881 g/cm3 |
| Boiling point | 224 to 225 °C (435 to 437 °F; 497 to 498 K) at 745 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Isomeric with nerol is geraniol, which is trans- or E-isomer. Nerol readily loses water to form a set of C10 compounds called dipentene. Nerol can be synthesized by pyrolysis of beta-pinene, which also affords myrcene. Hydrochlorination of myrcene gives a series of isomeric chlorides.
See also
References
- Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_141
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.