Neuromechanics
Neuromechanics is an interdisciplinary field that combines biomechanics and neuroscience to understand how the nervous system interacts with the skeletal and muscular systems to enable animals to move.[1][2] In a motor task, like reaching for an object, neural commands are sent to motor neurons to activate a set of muscles, called muscle synergies. Given which muscles are activated and how they are connected to the skeleton, there will be a corresponding and specific movement of the body.[3] In addition to participating in reflexes, neuromechanical process may also be shaped through motor adaptation and learning.[4]

Neuromechanics underlying behavior
Walking
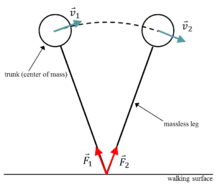
The inverted pendulum theory of gait is a neuromechanical approach to understand how humans walk. As the name of the theory implies, a walking human is modeled as an inverted pendulum consisting of a center of mass (COM) suspended above the ground via a support leg (Fig. 2). As the inverted pendulum swings forward, ground reaction forces occur between the modeled leg and the ground. Importantly, the magnitude of the ground reaction forces depends on the COM position and size. The velocity vector of the center of mass is always perpendicular to the ground reaction force.[5]
Walking consists of alternating single-support and double-support phases. The single-support phase occurs when one leg is in contact with the ground while the double-support phase occurs when two legs are in contact with the ground.[6]
Neurological influences
The inverted pendulum is stabilized by constant feedback from the brain and can operate even in the presence of sensory loss. In animals who have lost all sensory input to the moving limb, the variables produced by gait (center of mass acceleration, velocity of animal, and position of the animal) remain constant between both groups.[7]
During postural control, delayed feedback mechanisms are used in the temporal reproduction of task-level functions such as walking. The nervous system takes into account feedback from the center of mass acceleration, velocity, and position of an individual and utilizes the information to predict and plan future movements. Center of mass acceleration is essential in the feedback mechanism as this feedback takes place before any significant displacement data can be determined.[8]
Controversy
The inverted pendulum theory directly contradicts the six determinants of gait, another theory for gait analysis.[9] The six determinants of gait predict very high energy expenditure for the sinusoidal motion of the Center of Mass during gait, while the inverted pendulum theory offers the possibility that energy expenditure can be near zero; the inverted pendulum theory predicts that little to no work is required for walking.[5]
Measuring the neural control of muscles - Electromyography
Electromyography (EMG) is a tool used to measure the electrical outputs produced by skeletal muscles upon activation. Motor nerves innervate skeletal muscles and cause contraction upon command from the central nervous system. This contraction is measured by EMG and is typically measured on the scale of millivolts (mV). Another form of EMG data that is analyzed is integrated EMG (iEMG) data. iEMG measures the area under the EMG signal which corresponds to the overall muscle effort rather than the effort at a specific instant.
Equipment
There are four instrumentation components used to detect these signals: (1) the signal source, (2) the transducer used to detect the signal, (3) the amplifier, and (4) the signal processing circuit.[10] The signal source refers to the location at which the EMG electrode is place. EMG signal acquisition is dependent on distance from the electrode to the muscle fiber, so placement is imperative. The transducer used to detect the signal is an EMG electrode than transforms the bioelectric signal from the muscle to a readable electric signal.[10] The amplifier reproduces an undistorted bioelectric signal and also allows for noise reduction in the signal.[10] Signal processing involves taking the recorded electrical impulses, filtering them, and enveloping the data.[10]
Latency
Latency is a measure of the time span between the activation of a muscle and its peak EMG value. Latency is used as a means to diagnose disorders of the nervous system such as a herniated disc, amyotrophic lateral sclerosis (ALS), or myasthenia gravis (MG).[11] These disorders may cause a disruption of the signal at the muscle, the nerve, or the junction between the muscle and the nerve.
The use of EMG to identify nervous systems disorders is known as a nerve conduction study (NCS). Nerve conduction studies can only diagnose diseases on the muscular and nerve level. They cannot detect disease in the spinal cord or the brain. In most disorders of the muscle, nerve, or neuromuscular junction, the latency time is increased.[12] This is a result of decreased nerve conduction or electrical stimulation at the site of the muscle. In 50% of patients with cerebral atrophy cases, the M3 spinal reflex latency, was increased and on occasion separated from the M2 spinal reflex response.[13][14] The separation between the M2 and M3 spinal reflex responses is typically 20 milliseconds, but in patients with cerebral atrophy, the separation was increased to 50 ms. In some cases, however, other muscles can compensate for the muscle suffering from decreased electrical stimulation. In the compensatory muscle, the latency time is actually decreased in order to substitute for the function of the diseased muscle.[15] These kinds of studies are used in neuromechanics to identify motor disorders and their effects on a cellular and electrical level rather than a system motion level.
Coordinated movements enabled through muscle synergies
A muscle synergy is a group of synergistic muscles and agonists that work together to perform a motor task. A muscle synergy is composed of agonist and synergistic muscles. An agonist muscle is a muscle that contracts individually, and it can cause a cascade of motion in neighboring muscles. Synergistic muscles aid the agonist muscles in motor control tasks, but they act against excess motion that the agonists may create.
Muscle synergy hypothesis
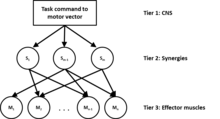
The muscle synergy hypothesis is based on the assumption that the central nervous system controls muscle groups independently rather than individual muscles .[16][17] The muscle synergy hypothesis presents motor control as a three-tiered hierarchy. In tier one, a motor task vector is created by the central nervous system. The central nervous system then transforms the muscle vector to act upon a group of muscle synergies in tier two. Then in tier three, muscle synergies define a specific ratio of the motor task for each muscle and assign it to its respective muscle to act upon the joint to perform the motor task.
Redundancy
Redundancy plays a large role in muscle synergy. Muscle redundancy is a degrees of freedom problem on the muscular level.[18] The central nervous system is presented with the opportunity to coordinate muscle movements, and it must choose one out of many. The muscle redundancy problem is a result of more muscle vectors than dimensions in the task space. Muscles can only generate tension by pulling, not pushing. This results in many muscle force vectors in multiple directions rather than a push and pull in the same direction.
One debate on muscle synergies is between the prime mover strategy and the cooperation strategy.[18] The prime mover strategy arises when a muscle's vector can act in the same direction as the mechanical action vector, the vector of the limb's motion. The cooperation strategy, however, takes place when no muscle can act directly in the vector direction of the mechanical action resulting in a coordination of multiple muscles to achieve the task. The prime mover strategy over time has declined in popularity as it has been found through electromyography studies that no one muscle consistently provides more force than other muscles that are acting to move about a joint.[19]
Criticisms
The muscle synergy theory is difficult to falsify.[20] Though experimentation has shown that groups of muscles indeed work together to control motor tasks, neural connections allow for individual muscles to be activated. Though individual muscle activation may contradict muscle synergy, it also obscures it. Activation of individual muscles may override or block the input from and overall effect of muscle synergies.[20]
Motor adaptation
Adaptation in the neuromechanical sense is the body's ability to change an action to better suit the situation or environment in which it is acting. Adaptation can be a result of injury, fatigue, or practice. Adaptation can be measured in a variety of ways: electromyography, three-dimensional reconstruction of joints, and changes in other variables pertaining to the specific adaptation being studied.
Injury
Injury can cause adaptation in a number of ways. Compensation is a large factor in injury adaptation. Compensation is a result of one or more weakened muscles. The brain is given the task to perform a certain motor task, and once a muscle has been weakened, the brain computes energy ratios to send to other muscles to perform the original task in the desired fashion. Change in muscle contribution is not the only byproduct of a muscle-related injury. Change in loading of the joint is another result which, if prolonged, can be harmful for the individual.[21]
Fatigue
Muscle fatigue is the neuromuscular adaptation to challenges over a period of time. The use of motor units over a period of time can result in changes in the motor command from the brain. Since the force of contraction cannot be changed, the brain instead recruits more motor units to achieve maximal muscle contraction.[22] Recruitment of motor units varies from muscle to muscle depending on the upper limit of motor recruitment in the muscle.[22]
Practice
Adaptation due to practice can be a result of intended practice such as sports or unintended practice such as wearing an orthosis. In athletes, repetition results in muscle memory. The motor task becomes a long-term memory that can be repeated without much conscious effort. This allows the athlete to focus on fine-tuning their motor task strategy. Resistance to fatigue also comes with practice as the muscle is strengthened, but the speed at which an athlete can complete a motor task is also increased with practice.[23] Volleyball players compared to non-jumpers show more repeatable control of muscles surrounding the knee that is controlled by co-activation in the single jump condition.[23] In the repeated jump condition, both volleyball players and non-jumpers have a linear decrease in normalized jump flight time.[23] Though the normalized linear decrease is the same for athletes and non-athletes, athletes consistently have higher flight times.
There is also adaptation associated with use of a prosthesis or an orthosis. This operates similarly to adaptation due to fatigue; however, muscles can actually be fatigued or alter their mechanical contribution to a motor task as a result of wearing the orthosis. An ankle foot orthosis is a common solution to injury of the lower limb, specifically around the ankle joint. An ankle foot orthosis can be assistive or resistive. An assistive ankle orthosis encourages ankle movement, and a resistive ankle orthosis inhibits ankle movement.[24] Upon wearing an assistive ankle foot orthosis, individuals have decreased EMG amplitude and joint stiffness over time while the opposite occurs for resistive ankle foot orthoses.[24] Additionally, not only can electromyography readings differ, but the physical path that joints travel along can be altered as well.[25]
References
- Enoka, Roger (1988). Neuromechanical Basis of Kinesiology. Human Kinetics. ISBN 978-0873221795.
- Nishikawa, K; Biewener, AA; Aerts, P; Ahn, AN; Chiel, HJ; Daley, MA; Daniel, TL; Full, RJ; Hale, ME; Hedrick, TL; Lappin, AK; Nichols, TR; Quinn, RD; Satterlie, RA; Szymik, B (July 2007). "Neuromechanics: an integrative approach for understanding motor control". Integrative and Comparative Biology. 47 (1): 16–54. doi:10.1093/icb/icm024. PMID 21672819.
- Constanzo, Linda (2013). Physiology. W B Saunders Co. ISBN 978-1455708475.
- Byl, NN (2004). "Focal hand dystonia may result from aberrant neuroplasticity". Advances in Neurology. 94: 19–28. PMID 14509650.
- Kuo, Arthur (6 July 2007). "The six determinants of gait and the inverted pendulum analogy: A dynamic walking perspective". Human Movement Science. 26 (4): 617–656. doi:10.1016/j.humov.2007.04.003. PMID 17617481.
- Kuo, Arthur; Donelan, Ruina (2005). "Energetic Consequences of Walking Like an Inverted Pendulum: Step-to-Step Transitions" (PDF). Exercise and Sport Sciences Reviews. 33 (2): 88–97. doi:10.1097/00003677-200504000-00006. PMID 15821430. S2CID 10566552.
- Lockhart, Daniel; Ting (16 September 2007). "Optimal sensorimotor transformations for balance". Nature Neuroscience. 10 (10): 1329–1336. doi:10.1038/nn1986. PMID 17873869. S2CID 18712682.
- Welch, Torrence; Ting (19 December 2007). "A Feedback Model Reproduces Muscle Activity During Human Postural Responses to Support-Surface Transitions". Journal of Neurophysiology. 99 (2): 1032–1038. doi:10.1152/jn.01110.2007. PMID 18094102.
- Cuccurullo, Sara (2009). Physical Medicine and Rehabilitation Board Review. Demos Medical Publishing. pp. 457–462. ISBN 978-1933864181.
- Soderberg, Gary; Cook (December 1984). "Electromyography in Biomechanics". Journal of the American Physical Therapy Association. 64 (12): 1813–1820. doi:10.1093/ptj/64.12.1813. PMID 6505026. Retrieved 10 November 2013.
- "Electromyogram (EMG) and Nerve Conduction Studies". WebMD, LLC. 1 March 2011. Retrieved 27 November 2013.
- Claus, Detlef; Schocklmann, Dietrich (1986). "Long Latency Muscle Responses in Cerebellar Diseases". European Archives of Psychiatry and Neurological Sciences. 235 (6): 355–360. doi:10.1007/bf00381004. PMID 3488906. S2CID 25660915.
- Claus, D; Schöcklmann, HO; Dietrich, HJ (1986). "Long latency muscle responses in cerebellar diseases". European Archives of Psychiatry and Neurological Sciences. 235 (6): 355–60. doi:10.1007/bf00381004. PMID 3488906. S2CID 25660915.
- Aminoff, [edited by] William F. Brown, Charles F. Bolton, Michael J. (2002). Neuromuscular function and disease : basic, clinical, and electrodiagnostic aspects (1st ed.). Philadelphia: W. B. Saunders company. pp. 229–230. ISBN 978-0721689227.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - Beckman, Scott; Buchanan (December 1995). "Ankle inversion injury and hypermobility: Effect on hip and ankle muscle electromyography onset latency". Archives of Physical Medicine and Rehabilitation. 76 (12): 1138–1143. doi:10.1016/s0003-9993(95)80123-5. PMID 8540791.
- Bernshtein, N. A (1967). The co-ordination and regulation of movements. New York: Pergamon Press. OCLC 301528509.
- Bizzi, E.; Cheung, V.C.K.; d'Avella, A.; Saltiel, P.; Tresch, M. (2008). "Combining modules for movement". Brain Research Reviews. 57 (1): 125–133. doi:10.1016/j.brainresrev.2007.08.004. PMC 4295773. PMID 18029291.
- Kutch, Jason (2008). "Signal in Human Motor Unsteadiness: Determining the Action and Activity of Muscles" (PDF). University of Michigan. Retrieved 8 November 2013.
- Buchanan, T.S.; Rovai, Rymer (1 December 1989). "Strategies for muscle activation during isometric torque generation at the human elbow". Journal of Neurophysiology. 62 (6): 1201–1212. doi:10.1152/jn.1989.62.6.1201. PMID 2600619.
- Tresch, Matthew C.; Jarc, A (December 2009). "The case for and against muscle synergies". Current Opinion in Neurobiology. 19 (6): 601–607. doi:10.1016/j.conb.2009.09.002. PMC 2818278. PMID 19828310.
- Liu, Wen; Maitland (29 October 1999). "The effect of hamstring muscle compensation for anterior laxity in the ACL-deficient knee during gait". Journal of Biomechanics. 33 (7): 871–879. doi:10.1016/s0021-9290(00)00047-6. PMID 10831762.
- Enoka, Roger; Stuart (1 May 1992). "Neurobiology of muscle fatigue". Journal of Applied Physiology. 72 (5): 1631–1648. doi:10.1152/jappl.1992.72.5.1631. PMID 1601767. S2CID 1572573. Retrieved 15 November 2013.
- Masci, Ilaria; Vannozzi, Gizzi; Bellotti, Felici (22 September 2009). "Neuromechanical evidence of improved neuromuscular control around knee joint in volleyball players". European Journal of Applied Physiology. 108 (3): 443–450. doi:10.1007/s00421-009-1226-z. PMID 19826834. S2CID 10986094.
- Chang, YH; Roiz, RA; Auyang, AG (2008). "Intralimb compensation strategy depends on the nature of joint perturbation in human hopping". Journal of Biomechanics. 41 (9): 1832–9. doi:10.1016/j.jbiomech.2008.04.006. PMID 18499112.
- Ferris, Daniel; Bohra; Lukos; Kinnaird (22 September 2005). "Neuromechanical adaptation to hopping with an elastic ankle-foot orthosis". Journal of Applied Physiology. 100 (1): 163–170. doi:10.1152/japplphysiol.00821.2005. PMID 16179395.
