Organonickel(IV) complex
Organonickel(IV) complex are organonickel compounds that feature nickel in the +4 oxidation state. These high-valent nickel compounds are intermediates or models thereof for various catalytic reactions.[1][2]
Representative preparation
Ni(IV) complexes are typically supported by highly basic and chelating ligands. They are often produced by oxidation of related Ni(II) and Ni(III) complexes using both conventional and exotic oxidants, such as ferrocenium and S-(trifluoromethyl)dibenzothiophenium triflate (Umemoto reagent). For example, one of the first isolated NiIV complexes that was used in a cross coupling transformation was reported by Linden and Dimitrov in 2003[3] and was prepared by simple air-induced oxidation of the following anionic NiII complex:
_to_Ni(IV).png.webp)
Reactivity and synthetic transformations
Carbon-heteroatom bond formation
Studies reported by Sanford and Camasso in 2015 revealed the ability of a NiIV organometallic complex to deliver carbon(sp3)-oxygen, carbon(sp3)-nitrogen, and carbon(sp3)-sulfur bonds. The NiIV species was generated by oxidation of a nickel(II) trispyrazolylborate complex.[4]
_2015.png.webp)
C-H activation
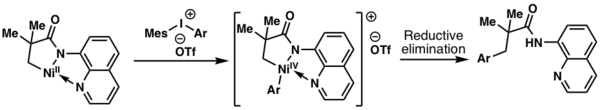
In 2014, Chatani and coworkers proposed that a NiIV intermediate was formed during an aminoquinoline-directed aliphatic C-H activation process using diaryliodonium salts as an oxidant.[5]
Trifluoromethylations
NiIV can perform challenging trifluoromethylation reactions on (hetero)arenes. A representative method is shown below, reported by Nebra and coworkers in 2017. Here, a six-coordinate NiIV complex transfers a CF3 ligand to a functionalized arene.[6]
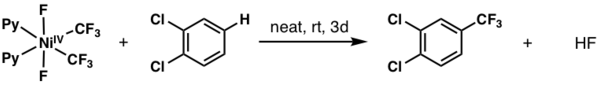
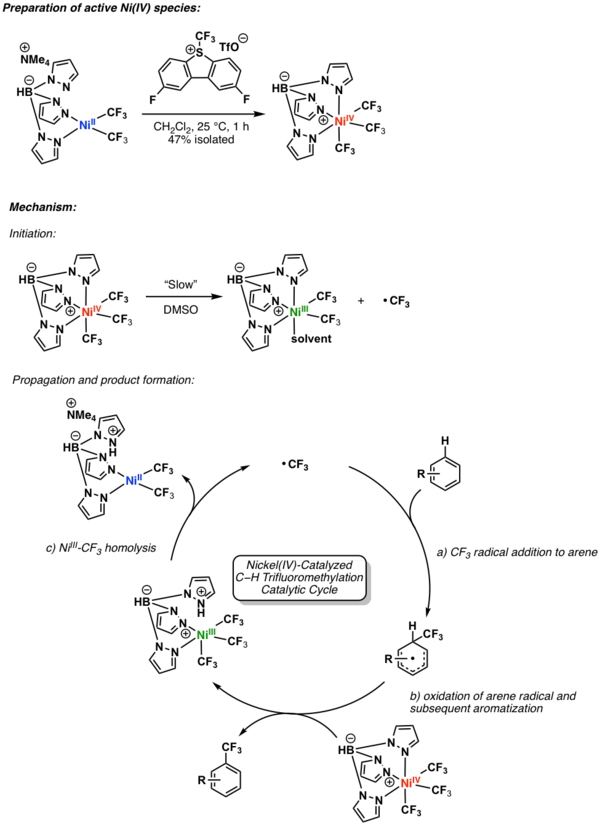
A catalytic variant was reported by Sanford and coworkers in 2019. The mechanism for this transformation is shown in the following scheme. Here, the key NiIV intermediate undergoes a slow homolysis to produce a NiIII intermediate and a trifluoromethyl radical, which can promote propagation of the catalytic cycle.[7]
References
- Nebra, Noel (2020-03-04). "High-Valent NiIII and NiIV Species Relevant to C–C and C–Heteroatom Cross-Coupling Reactions: State of the Art". Molecules. 25 (5): 1141. doi:10.3390/molecules25051141. ISSN 1420-3049. PMC 7179250. PMID 32143336.
- Diccianni, Justin B.; Diao, Tianning (December 2019). "Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions". Trends in Chemistry. 1 (9): 830–844. doi:10.1016/j.trechm.2019.08.004. ISSN 2589-5974. S2CID 203131989.
- Dimitrov, Vladimir; Linden, Anthony (2003-06-16). "A Pseudotetrahedral, High-Oxidation-State Organonickel Compound: Synthesis and Structure of Bromotris(1-norbornyl)nickel(IV)". Angewandte Chemie International Edition. 42 (23): 2631–2633. doi:10.1002/anie.200219383. ISSN 1433-7851. PMID 12813738.
- Camasso, N. M.; Sanford, M. S. (2015-02-05). "Design, synthesis, and carbon-heteroatom coupling reactions of organometallic nickel(IV) complexes". Science. 347 (6227): 1218–1220. Bibcode:2015Sci...347.1218C. doi:10.1126/science.aaa4526. ISSN 0036-8075. PMID 25766226. S2CID 206634533.
- Iyanaga, Miki; Aihara, Yoshinori; Chatani, Naoto (May 2015). "ChemInform Abstract: Direct Arylation of C(sp3)-H Bonds in Aliphatic Amides with Diaryliodonium Salts in the Presence of a Nickel Catalyst". ChemInform. 46 (21): no. doi:10.1002/chin.201521038. ISSN 0931-7597.
- D'Accriscio, Florian; Borja, Pilar; Saffon-Merceron, Nathalie; Fustier-Boutignon, Marie; Mézailles, Nicolas; Nebra, Noel (2017-09-06). "C−H Bond Trifluoromethylation of Arenes Enabled by a Robust, High-Valent Nickel(IV) Complex". Angewandte Chemie International Edition. 56 (42): 12898–12902. doi:10.1002/anie.201706237. ISSN 1433-7851. PMID 28815889.
- Meucci, Elizabeth A.; Nguyen, Shay N.; Camasso, Nicole M.; Chong, Eugene; Ariafard, Alireza; Canty, Allan J.; Sanford, Melanie S. (2019-08-05). "Nickel(IV)-Catalyzed C–H Trifluoromethylation of (Hetero)arenes". Journal of the American Chemical Society. 141 (32): 12872–12879. doi:10.1021/jacs.9b06383. ISSN 0002-7863. PMC 7542544. PMID 31379153.