Nirogacestat
Nirogacestat (PF-03084014) is a selective gamma secretase inhibitor[1] developed by SpringWorks Therapeutics that has potential anti-tumor activity. It was granted FDA breakthrough drug designation in September 2019 for adult patients with progressive, unresectable, recurrent or refractory desmoid tumors or deep fibromatosis.[2]
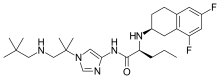 | |
| Clinical data | |
|---|---|
| Other names | PF-03084014 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H41F2N5O |
| Molar mass | 489.656 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medical uses
As of 2021, nirogacestat was in Phase 2 clinical trials for unresectable desmoid tumors.[3] In addition, a Phase 3 clinical trial, DeFi, was in progress for nirogacestat for adults with desmoid tumors and aggressive fibromatosis.[4] In addition, three trials were recruiting patients that include nirogacestat with other anticancer therapies in multiple myeloma, including the UNIVERSAL study for nirogacestat with the allogeneic CAR-T therapy ALLO-715.[5][6][7]
References
- Chen X, Chen X, Zhou Z, Mao Y, Wang Y, Ma Z, Xu W, Qin A, Zhang S (September 2019). "Nirogacestat suppresses RANKL-Induced osteoclast formation in vitro and attenuates LPS-Induced bone resorption in vivo". Experimental Cell Research. 382 (1): 111470. doi:10.1016/j.yexcr.2019.06.015. PMID 31211955. S2CID 195065514.
- "FDA Grants Nirogacestat Breakthrough Designation for Desmoid Tumors". OncLive. Retrieved 2021-06-25.
- Clinical trial number NCT04195399 for "A Safety, Pharmacokinetic and Efficacy Study of a y-Secretase Inhibitor, Nirogacestat (PF-03084014) in Children and Adolescents With Progressive, Surgically Unresectable Desmoid Tumors" at ClinicalTrials.gov
- Clinical trial number NCT03785964 for "A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial of Nirogacestat Versus Placebo in Adult Patients With Progressing Desmoid Tumors/Aggressive Fibromatosis (DT/AF)" at ClinicalTrials.gov
- Clinical trial number NCT04093596 for "A Single-Arm, Open-Label, Phase 1 Study of the Safety, Efficacy, and Cellular Kinetics/Pharmacodynamics of ALLO-715 to Evaluate an Anti-BCMA Allogeneic CAR T Cell Therapy With or Without Nirogacestat in Subjects With Relapsed/Refractory Multiple Myeloma" at ClinicalTrials.gov
- Clinical trial number NCT04722146 for "A Multi-arm Phase 1b Study of Teclistamab With Other Anticancer Therapies in Participants With Multiple Myeloma" at ClinicalTrials.gov
- Clinical trial number NCT04126200 for "A Phase I/II, Randomized, Open-label Platform Study Utilizing a Master Protocol to Study Belantamab Mafodotin (GSK2857916) as Monotherapy and in Combination With Anti-Cancer Treatments in Participants With Relapsed/Refractory Multiple Myeloma (RRMM) - DREAMM 5" at ClinicalTrials.gov