Nitensidine D
Nitensidine D is a toxic alkaloid natural product that was isolated from the leaves of the South American legume Pterogyne nitens.[2] It is also hypothesized to be a possible intermediate in the still unknown, seemingly monoterpene based, terrestrial biosynthetic pathway for tetrodotoxin.[3]
 | |
| Names | |
|---|---|
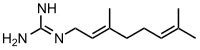
| IUPAC name
(E)-2-(3,7-dimethylocta-2,6-dien-1-yl)guanidine | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C11H21N3 | |
| Molar mass | 195.310 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- "KNApSAcK Metabolite Information - C00047317". www.knapsackfamily.com.
- Regasini, Luis Octávio; Castro-Gamboa, Ian; Silva, Dulce Helena Siqueira; Furlan, Maysa; Barreiro, Eliezer Jesus; Ferreira, Paulo Michel Pinheiro; Pessoa, Cláudia; Lotufo, Letícia Veras Costa; de Moraes, Manoel Odorico; Young, Maria Claudia Marx; Bolzani, Vanderlan da Silva (2009-03-27). "Cytotoxic Guanidine Alkaloids fromPterogyne nitens△". Journal of Natural Products. American Chemical Society (ACS). 72 (3): 473–476. doi:10.1021/np800612x. ISSN 0163-3864. PMID 19159272.
- Kudo, Yuta; Yotsu-Yamashita, Mari (2019-05-22). "Isolation and Biological Activity of 8-Epitetrodotoxin and the Structure of a Possible Biosynthetic Shunt Product of Tetrodotoxin, Cep-226A, from the Newt Cynops ensicauda popei". Journal of Natural Products. American Chemical Society (ACS). 82 (6): 1656–1663. doi:10.1021/acs.jnatprod.9b00178. ISSN 0163-3864. PMID 31117524. S2CID 162180379.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.