Nitramide
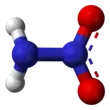
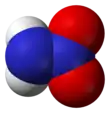
Nitramide is a chemical compound with the molecular formula H2NNO2. Organyl derivatives of nitramide, RNHNO2 are termed nitroamines, and are widely used as explosives: examples include RDX and HMX. It is an isomer of hyponitrous acid.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Nitramine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| H2N2O2 | |||
| Molar mass | 62.03 g mol−1 | ||
| Appearance | Colorless solid[1] | ||
| Density | 1.378 g/cm3 | ||
| Melting point | 72 to 75 °C (162 to 167 °F; 345 to 348 K)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Structure
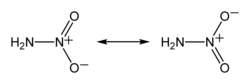
The nitramide molecule is essentially an amine group (−NH2) bonded to a nitro group (−NO2). It is reported to be non-planar in the gas phase,[2] but planar in the crystal phase.[1]
Synthesis
Thiele and Lachman's original synthesis of nitramide involved the hydrolysis of potassium nitrocarbamate:[1]
Other routes to nitramide include hydrolysis of nitrocarbamic acid,
reaction of sodium sulfamate with nitric acid,
and reaction of dinitrogen pentoxide with two equivalents of ammonia.
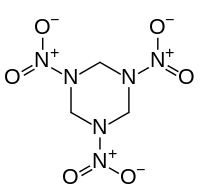
Organic nitramides
Also called nitramines, organic nitramides are important explosives. They are prepared by nitrolysis of hexamethylenetetramine.
References
- Häußler, A.; Klapötke, T. M.; Piotrowski, H. (2002). "Experimental and Theoretical Study on the Structure of Nitramide H2NNO2" (PDF). Zeitschrift für Naturforschung. 57 b (2): 151–156.
- Tyler, J. K. (1963). "Microwave Spectrum of Nitramide". Journal of Molecular Spectroscopy. 11 (1–6): 39–46. doi:10.1016/0022-2852(63)90004-3.