Nitroalkene
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.[1]
Synthesis
Nitroalkenes are synthesized by various means, notable examples include:
- Nitroaldol reactions such as the Henry reaction:[1][2][3][4]
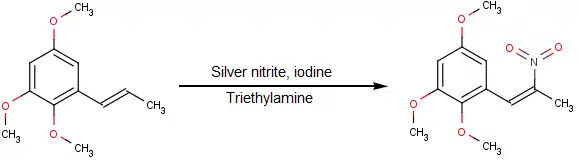
- Nitration of an alkene with nitryl iodide generated in-situ from silver nitrite and elemental iodine:[5]
- Direct nitration of alkenes with nitric oxide and an aluminum oxide catalyst in acidic conditions:[6]

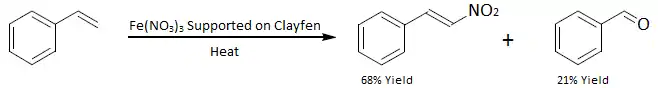
- Direct nitration of alkenes with Clayfen (Iron(III) nitrate supported on Montmorillonite clay):[7]
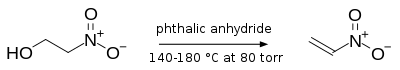
- Dehydration of nitro-alcohols:[8]
Reactions
Nitroalkenes are useful intermediates for various chemical functionalities.
- A nitroalkene behaving as a Michael acceptor in the synthesis of Lycoricidine:[1][9]
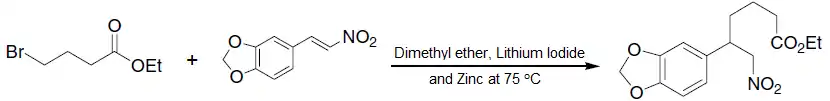
- Nitroalkene acting as an activated dienophile toward butadiene in a Diels-Alder cycloaddition:[1][10]
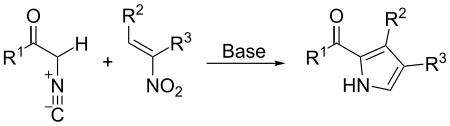
- The synthesis of pyrrole derivatives via the Barton–Zard reaction:[11]
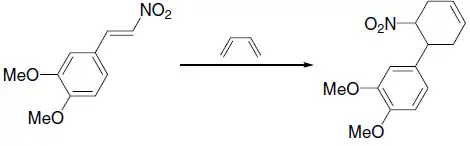
- Pericyclic reaction of a nitroalkene yielding an indole:[12]
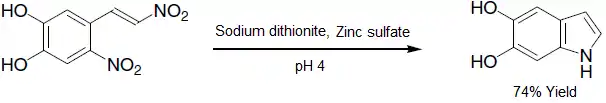
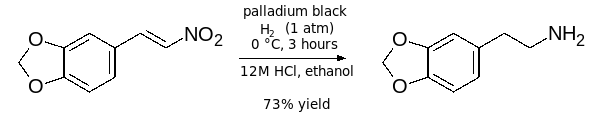
- Partial hydrogenation to an alkene baring a hydroxylamine functional group:[13]

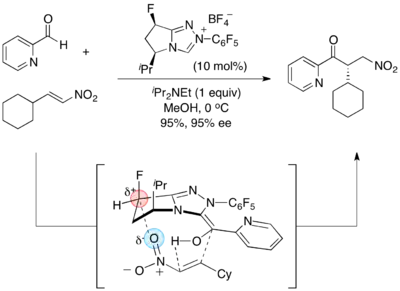
- Asymmetric Stetter reaction:[15]
References
- Furniss, Brian; Hannaford, Antony; Smith, Peter & Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed. London: Longman Science & Technical. pp. 635, 768, 1035–1036, & 1121. ISBN 9780582462366.
- Ballini, Roberto; Castagnani, Roberto; Petrini, Marino (1992). "Chemoselective synthesis of functionalized conjugated nitroalkenes". The Journal of Organic Chemistry. 57 (7): 2160–2162. doi:10.1021/jo00033a045.
- Worrall, David E. (1929). "Nitrostyrene". Org. Synth. 9: 66. doi:10.15227/orgsyn.009.0066.
- Chandrasekhar, S.; Shrinidhi, A. (2014). "Useful Extensions of the Henry Reaction: Expeditious Routes to Nitroalkanes and Nitroalkenes in Aqueous Media". Synthetic Communications. 44 (20): 3008–3018. doi:10.1080/00397911.2014.926373. S2CID 98439096.
- Waldman, Steve; Monte, Aaron, Monte; Bracey, Ann & Nichols, David (1996). "One-pot Claisen rearrangement/O-methylation/alkene isomerization in the synthesis of ortho-methoxylated phenylisopropylamines". Tetrahedron Letters. 37 (44): 7889–7892. doi:10.1016/0040-4039(96)01807-2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Mukaiyama, T.; Hata E. & Yamada, T. (1995). "Convenient and Simple Preparation of Nitroolefins Nitration of Olefins with Nitric Oxide". Chemistry Letters. 24 (7): 505–506. doi:10.1246/cl.1995.505.
- Varma, Rajender; Naicker, Kannan; Liesen, Per (1998). "Selective nitration of styrenes with clayfen and clayan: A solvent-free synthesis of β-nitrostyrenes". Tetrahedron Letters. 39 (23): 3977–3980. doi:10.1016/S0040-4039(98)00740-0.
- Ranganathan, Darshan; Rao, Bhushan; Ranganathan, Subramania; Mehrotra, Ashok & Iyengar, Radha (1980). "Nitroethylene: a stable, clean, and reactive agent for organic synthesis". The Journal of Organic Chemistry. 45 (7): 1185–1189. doi:10.1021/jo01295a003.
- Jubert, Carole & Knochel, Paul (1992). "Preparation of polyfunctional nitro olefins and nitroalkanes using the copper-zinc reagents RCu(CN)ZnI". The Journal of Organic Chemistry. 57 (20): 5431–5438. doi:10.1021/jo00046a027.
- Noboru Ono; Hideyoshi Miyake; Akio Kamimura & Aritsune, Kaji (1987). "Regioselective Diels–Alder reactions. The nitro group as a regiochemical control element". Perkin Transactions. 1: 1929–1935. doi:10.1039/P19870001929.
- Jie Jack Li (2013). Heterocyclic Chemistry in Drug Discovery. New York: Wiley. ISBN 9781118354421. pp.43-4
- Novellino, Luisa; d'Ischia, Marco & Prota, Giuseppe (1999). "Expedient Synthesis of 5,6-Dihydroxyindole and Derivatives via an Improved Zn(II)-Assisted 2,β-Dinitrostyrene Approach". Synthesis. 1999 (5): 793–796. doi:10.1055/s-1999-3469.
- Masahiko Kohno; Shigehiro Sasao & Shun-Ichi Murahashi (1990). "Synthesis of Phenethylamines by Hydrogenation of β-Nitrostyrenes". Bulletin of the Chemical Society of Japan. 63 (4): 1252–1254. doi:10.1246/bcsj.63.1252.
- Koch, Werner & Reichert, Benno (1935). "Über die katalytische Hydrierung substituierter ω-Nitrostyrole". Archiv der Pharmazie. 273 (18–20): 265–274. doi:10.1002/ardp.19352731802. S2CID 95731916.
- DiRocco, D. A.; Oberg, K. M.; Dalton, D. M.; Rovis, T. (2009). "Catalytic Asymmetric Intermolecular Stetter Reaction of Heterocyclic Aldehydes with Nitroalkenes: Backbone Fluorination Improves Selectivity". Journal of the American Chemical Society. 131 (31): 10872–10874. doi:10.1021/ja904375q. PMC 2747345. PMID 19722669.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
