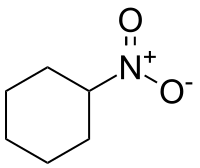
Nitrocyclohexane
Nitrocyclohexane is an organic compound with the molecular formula C6H11NO2. It is a colorless liquid, but degraded samples appear pale yellow.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Nitrocyclohexane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.050 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H11NO2 | |
| Molar mass | 129.159 g·mol−1 |
| Density | 1.061 g/cm3 |
| Melting point | −34 °C (−29 °F; 239 K) |
| Boiling point | 205.8 °C (402.4 °F; 478.9 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
It is prepared by reaction of nitrogen dioxide with cyclohexane. Cyclohexane is a convenient substrate because all twelve C-H bonds are equivalent, so mononitration does not give isomers (unlike the case of n-hexane).[1]
Hazards
Nitrocyclohexane is highly flammable and a strong oxidizing agent.[2] It is listed as an extremely hazardous substance by the Emergency Planning and Community Right-to-Know Act, and the NOAA warns that it can be explosive.[2]
References
- Sakaguchi, Satoshi; Nishiwaki, Yoshiki; Kitamura, Takaaki; Ishii, Yasutaka (2001). "Efficient Catalytic Alkane Nitration with NO2 under Air Assisted by N-Hydroxyphthalimide". Angewandte Chemie International Edition. 40: 222–224. doi:10.1002/1521-3773(20010105)40:1<222::AID-ANIE222>3.0.CO;2-W.
- "Cameo Chemicals - NITROCYCLOHEXANE". Retrieved July 9, 2012.
Further reading
- Iffland, Don C.; Criner, G. X. (1953). "Preparation of Nitro Compounds from Oximes. II. The Improved Synthesis of Nitrocycloalkanes1". Journal of the American Chemical Society. 75 (16): 4047. doi:10.1021/ja01112a049.
- Fahim, Hussein; Fleifel, Abdallah; Fahim, Fawzia (1960). "Synthesis of 1-Benzylnaphthalenes". The Journal of Organic Chemistry. 25 (6): 1040–1041. doi:10.1021/jo01076a605.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.