Nitrosylation
Nitrosylation is the general term for covalent incorporation of a nitric oxide "nitrosyl" moiety into another (usually organic) molecule. There are multiple chemical mechanisms by which this can be achieved; including biological enzymes and industrial processes. The biological functions of nitrosylation are particularly important as S-nitrosylation, the conjugation of NO to cysteine thiols in proteins, is an important part of cell signalling.[1] Coordination of NO to transition metals to give metal nitrosyl complexes, is also referred to as nitrosylation.[2]
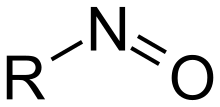
Nitrosylation results in a molecule "R" adducted with the group N=O
See also
References
- Mannick, Joan B.; Schonhoff, Christopher M. (7 July 2009). "Review: NO Means No and Yes: Regulation of Cell Signaling by Protein Nitrosylation". Free Radical Research. 38 (1): 1–7. doi:10.1080/10715760310001629065. PMID 15061648. S2CID 21787778.
- Hayton, T. W.; Legzdins, P.; Sharp, W. B. (2002). "Coordination and Organometallic Chemistry of Metal-NO Complexes". Chem. Rev. 102 (1): 935–991. doi:10.1021/cr000074t. PMID 11942784.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.