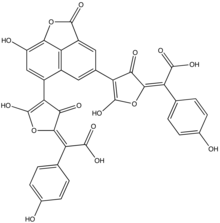
Norbadione A
Norbadione A is a pigment found in the bay bolete mushroom (Boletus badius). A polyphenol, norbadione A is related to a family of mushroom pigments known as pulvinic acids.[1] The molecule has also been reported as a potassium salt from the mushrooms Pisolithus tinctorius (horse dung fungus)[2] and Chalciporus piperatus.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
[2(32)E,52(6)E]-14,33,48,53,74-Pentahydroxy-35,42,55-trioxo-35H,42H,55H-4(4,6)-naphtho[1,8-bc]furana-3(2,4),5(4,2)-difurana-1,7(1)-dibenzenaheptaphane-2(32),52(6)-diene-2,6-dicarboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| Properties | |
| C35H18O15 | |
| Molar mass | 678.50842 gmol−1 |
| Appearance | red needles |
| Density | 1.902 g/cm3 |
| Melting point | 300 °C (572 °F; 573 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Properties
Norbadione A has seven acid-base functional groups, among which are two enolic and two carboxylic acid moieties.[4] These functional groups confer water-solubility to the molecule. It selectively complexes cesium cations (Cs+),[5] with an efficiency comparable to that of some calixarenes or crown ethers.[4] It has been investigated for its ability to provide a protective effect against the damaging effects of ionizing radiation, an effect attributed to its ability to protect DNA-related targets from irradiation.[6] Tests with cell cultures and mice show that although it has some protective effect, it is toxic to cells in higher doses.[7] A diverse array of synthetic derivatives of norbadione A has been created to explore the effect of structure on antioxidant properties and cytotoxicity.[8] A series of alkali chelators based on the structure of norbadione A has been reported.[9] The intramolecular protonation process has been determined. There is a pH-dependent Z to E isomer switch that occurs in both pulvinate moieties,[10] which yields four stereoisomeric forms (E/E, E/Z, Z/Z, Z/E). These stereoisomers may have a widely differing ability to form complexes with Cs+ in solution.[6]
Synthesis
Bourdreux and colleagues reported a total synthesis of norbadione A in 2008. The technique uses a regioselective Diels–Alder reaction and a double Suzuki-Miyaura cross-coupling.[11]
References
- Aumann DC, Clooth G, Steffan B, Steglich W (1989). "Complexation of cesium-137 by the cap pigments of the bay boletus (Xerocomus badius)". Angewandte Chemie International Edition in English. 28 (4): 453–454. doi:10.1002/anie.198904531.
- Thompson RH (1997). Naturally Occurring Quinones IV. Springer. p. 282. ISBN 978-0-7514-0248-3.
- Yannai S. (2013). Dictionary of Food Compounds. CRC Press. p. 1416. ISBN 978-1-4200-8351-4.
- Korovitch A, Mulon JB, Souchon V, Leray I, Valeur B, Mallinger A, Nadal B, Le Gall T, Lion C, Ha-Duong NT, El Hage Chahine JM (2010). "Norbadione A: kinetics and thermodynamics of cesium uptake in aqueous and alcoholic media". Journal of Physical Chemistry B. 114 (39): 12655–12665. doi:10.1021/jp1060232. PMID 20831226.
- Kuad P, Schurhammer R, Maechling C, Antheaume C, Mioskowski C, Wipff G, Spiess B (2009). "Complexation of Cs+, K+ and Na+ by norbadione A triggered by the release of a strong hydrogen bond: nature and stability of the complexes". Physical Chemistry Chemical Physics. 11 (44): 10299–10310. Bibcode:2009PCCP...1110299K. doi:10.1039/b912518c. PMID 19890513.
- Schurhammer R, Diss R, Spiess B, Wipff G (2007). "Conformational and Cs+ complexation properties of norbadione-A: A molecular modeling study". Physical Chemistry Chemical Physics. 10 (4): 495–505. doi:10.1039/B712836C. PMID 18183312.
- Le Roux A, Josset E, Benzina S, Nadal B, Desage-El Murr M, Heurtaux B, Taran F, Denis J-M, Le Gall T, Meunier S, Bischoff P (2012). "Evaluation of the radioprotective potential of the polyphenol norbadione A". Letters in Drug Design & Discovery. 9 (1): 48–53. doi:10.2174/157018012798192900.
- Habrant D, Poigny S, Ségur-Derai M, Brunel Y, Heurtaux B, Le Gall T, Strehle A, Saladin R, Meunier S, Mioskowski C, Wagner A (2009). "Evaluation of antioxidant properties of monoaromatic derivatives of pulvinic acids". Journal of Medicinal Chemistry. 52 (8): 2454–2464. doi:10.1021/jm801500h. PMID 19309153.
- Korovitch A, Le Roux A, Barbault F, Hémadi M, Ha-Duong N-T, Lion C, Wagner A, El Hage Chahine J-M (2013). "A new series of Cs+, K+ and Na+ chelators: Synthesis, kinetics, thermodynamics and modeling". Inorganica Chimica Acta. 394: 45–57. doi:10.1016/j.ica.2012.08.009.
- Kuad P, Borkovec M, Desage-El Murr M, Le Gall T, Mioskowski C, Spiess B (2005). "Inframolecular protonation process of norbadione A: Influence of the ionic environment and stereochemical consequences". Journal of the American Chemical Society. 127 (4): 1323–1333. doi:10.1021/ja0483185. PMID 15669874.
- Bourdreux Y, Nowaczyk S, Billaud C, Mallinger A, Willis C, Murr MD, Toupet L, Lion C, Gall TL, Mioskowski C (2008). "Total synthesis of norbadione A". Journal of Organic Chemistry. 73 (1): 22–26. doi:10.1021/jo702106u. PMID 18052074.