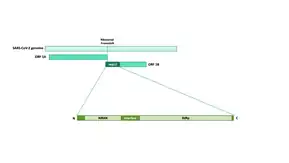
Nsp12
Nsp12 is a non-structural protein in the Coronavirus genome. Its gene is part of the ORF1ab reading frame and it is part of the pp1ab polyprotein; it is cleaved by 3CLpro.[1]
Nsp12 is a multi-domain subunit: it consists of an N-terminal nidovirus-specific extension (NiRAN) domain, an interface domain, and a C-terminal RNA-dependent RNA-polymerase domain. The N-terminal portion of SARS-CoV-2 nsp12 additionally contains a β-hairpin which is sandwiched between the NiRAN and RdRp domain.[2]
Coronavirus nsp12 also plays a role in host immune evasion; research has demonstrated that nsp12 inhibits the nuclear translocation of IRF3.[3]
RdRp Domain
The RNA-dependent RNA polymerase domain of nsp12 is C-terminal. In SARS-CoV-2 the domain spans residues 366 to 920.[4] The structure of the RdRp domain shares common structural features with eukaryotic RNA polymerases: the structure consists of a cupped right hand with subdomains referred to as fingers, palms, and thumbs.[1] RdRp activity is dependent on two key zinc ions and conserved metal binding motifs of a histidine and two cysteines each.[2]
The active site has seven catalytic motifs that are labeled A through G. Motif B serves as a hinge which allows the active site to associate with template RNA and Motif F directly interacts with the phosphate group of incoming free nucleotides.[2]
RdRp has to interact with RNA, which is negatively charged, so multiple subdomains including the primer-template entry site, NTP entry site, and the RNA strand exit routes contain positively charged residues.[2] RdRp is unique from host RNA polymerases in that it has to associate with RNA instead of DNA, many RdRp residues interact with RNA bases via 2’-OH groups on the ribose ring which provides a possibly structural explanation for its specificity for RNA.[4]
Coronavirus nsp12 cannot function independently; it has two essential cofactor proteins, nsp7 and nsp8, that form a Replication and Transcription Complex (RTC).[5] Structural studies of the RTC indicate that nsp7 and nsp8 form an 8:8 hexadecamer which acts as a primase to initiate viral replication.[6]
While nsp12 is relatively well conserved across the Coronavirus viral species, there are biochemical and structural differences between the RdRp domain of SARS-CoV and SARS-CoV-2. SARS-CoV-2 RdRp has lower enzymatic activity and lower thermal stability compared to the RdRp domain in SARS-CoV.[7]
Targeting by Remdesivir
Nsp12 is researched as a target for antiviral drugs as it is highly structurally conserved across related viruses and strains, and there are no human proteins with close structural homology.[2] The emergence of SARS-CoV-2 and associated COVID19 disease led to the investigation of Remdesivir as an antiviral drug for SARS-CoV-2. Remdesivir is a nucleoside analog which can compete with ATP for incorporation into the RNA strand and prematurely terminate RNA synthesis.[5]
NiRAN Domain
Coronavirus nsp12 has an N-terminal nidovirus RdRp-associated nucleotidyltransferase (NiRAN) domain which is essential for viral replication. The NiRAN domain is capable of transferring nucleotides as functional groups and it contains three key motifs called A, B, and C with seven invariant residues.[8]
The biological function of the nsp12 NiRAN domain is not as well characterized as RdRp, but recent research has elucidated a possible role for the NiRAN domain in viral RNA capping. An additional non-structural protein, nsp9, was shown to associate with nsp12.[9] The biologically active form of nsp9 was additionally shown to be capable of binding nucleic acids with a preference for single-stranded RNA[10] and could cleave nucleotide triphosphates and transfer the resulting nucleotide monophosphates to protein substrates in a process called NMPylation.[9] Park and colleagues demonstrated that the SARS-CoV-2 NiRAN domain could cleave a pyrophosphate from the end of an uncapped RNA genome and transfer the monophosphorylated RNA to nsp9 to RNAylate it.[11] The domain can then transfer the monophosphorylated RNA from nsp9 to a Guanidine Diphosphate (GDP) to form the initial cap structure for SARS-CoV-2.[11]
References
- Snijder, E.J.; Decroly, E.; Ziebuhr, J. (2016), "The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing", Advances in Virus Research, Elsevier, 96: 59–126, doi:10.1016/bs.aivir.2016.08.008, ISBN 978-0-12-804736-1, PMC 7112286, PMID 27712628
- Jiang, Yi; Yin, Wanchao; Xu, H. Eric (2021-01-29). "RNA-dependent RNA polymerase: Structure, mechanism, and drug discovery for COVID-19". Biochemical and Biophysical Research Communications. COVID-19. 538: 47–53. doi:10.1016/j.bbrc.2020.08.116. ISSN 0006-291X. PMC 7473028. PMID 32943188.
- Wang, Wenjing; Zhou, Zhuo; Xiao, Xia; Tian, Zhongqin; Dong, Xiaojing; Wang, Conghui; Li, Li; Ren, Lili; Lei, Xiaobo; Xiang, Zichun; Wang, Jianwei (April 2021). "SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation". Cellular & Molecular Immunology. 18 (4): 945–953. doi:10.1038/s41423-020-00619-y. ISSN 1672-7681. PMC 7907794. PMID 33637958.
- Yin, Wanchao; Mao, Chunyou; Luan, Xiaodong; Shen, Dan-Dan; Shen, Qingya; Su, Haixia; Wang, Xiaoxi; Zhou, Fulai; Zhao, Wenfeng; Gao, Minqi; Chang, Shenghai; Xie, Yuan-Chao; Tian, Guanghui; Jiang, He-Wei; Tao, Sheng-Ce (2020-06-26). "Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir". Science. 368 (6498): 1499–1504. Bibcode:2020Sci...368.1499Y. doi:10.1126/science.abc1560. ISSN 0036-8075. PMC 7199908. PMID 32358203.
- Ionescu, Mihaela Ileana (2020-12-01). "An Overview of the Crystallized Structures of the SARS-CoV-2". The Protein Journal. 39 (6): 600–618. doi:10.1007/s10930-020-09933-w. ISSN 1875-8355. PMC 7584483. PMID 33098476.
- Zhai, Yujia; Sun, Fei; Li, Xuemei; Pang, Hai; Xu, Xiaoling; Bartlam, Mark; Rao, Zihe (November 2005). "Insights into SARS-CoV transcription and replication from the structure of the nsp7–nsp8 hexadecamer". Nature Structural & Molecular Biology. 12 (11): 980–986. doi:10.1038/nsmb999. ISSN 1545-9993. PMC 7096913. PMID 16228002.
- Peng, Qi; Peng, Ruchao; Yuan, Bin; Zhao, Jingru; Wang, Min; Wang, Xixi; Wang, Qian; Sun, Yan; Fan, Zheng; Qi, Jianxun; Gao, George F.; Shi, Yi (2020-06-16). "Structural and Biochemical Characterization of the nsp12-nsp7-nsp8 Core Polymerase Complex from SARS-CoV-2". Cell Reports. 31 (11): 107774. doi:10.1016/j.celrep.2020.107774. ISSN 2211-1247. PMC 7260489. PMID 32531208.
- Gorbalenya, Alexander E.; Enjuanes, Luis; Ziebuhr, John; Snijder, Eric J. (April 2006). "Nidovirales: Evolving the largest RNA virus genome". Virus Research. 117 (1): 17–37. doi:10.1016/j.virusres.2006.01.017. PMC 7114179. PMID 16503362.
- Slanina, Heiko; Madhugiri, Ramakanth; Bylapudi, Ganesh; Schultheiß, Karin; Karl, Nadja; Gulyaeva, Anastasia; Gorbalenya, Alexander E.; Linne, Uwe; Ziebuhr, John (2021-02-09). "Coronavirus replication–transcription complex: Vital and selective NMPylation of a conserved site in nsp9 by the NiRAN-RdRp subunit". Proceedings of the National Academy of Sciences. 118 (6): e2022310118. Bibcode:2021PNAS..11822310S. doi:10.1073/pnas.2022310118. ISSN 0027-8424. PMC 8017715. PMID 33472860.
- Ponnusamy, Rajesh; Moll, Ralf; Weimar, Thomas; Mesters, Jeroen R.; Hilgenfeld, Rolf (2008-11-28). "Variable Oligomerization Modes in Coronavirus Non-structural Protein 9". Journal of Molecular Biology. 383 (5): 1081–1096. doi:10.1016/j.jmb.2008.07.071. ISSN 0022-2836. PMC 7094590. PMID 18694760.
- Park, Gina J.; Osinski, Adam; Hernandez, Genaro; Eitson, Jennifer L.; Majumdar, Abir; Tonelli, Marco; Henzler-Wildman, Katie; Pawłowski, Krzysztof; Chen, Zhe; Li, Yang; Schoggins, John W.; Tagliabracci, Vincent S. (2022-08-09). "The mechanism of RNA capping by SARS-CoV-2". Nature. 609 (7928): 793–800. Bibcode:2022Natur.609..793P. doi:10.1038/s41586-022-05185-z. ISSN 0028-0836. PMC 9492545. PMID 35944563.