o-Anisic acid
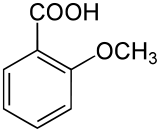
o-Anisic acid is an organic compound with the formula CH3OC6H4CO2H. A colorless solid, it is one of the isomers of anisic acid.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methoxybenzoic acid | |
| Other names
o-Anisic acid, , ortho-methoxybenzoic acid, 2-methoxybenzoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.590 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H8O3 | |
| Molar mass | 152.15 g/mol |
| Melting point | 101 to 103 °C (214 to 217 °F; 374 to 376 K) |
| Acidity (pKa) | 4.09[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The compound has been well studied with respect to intramolecular hydrogen bonding[2] and as a substrate for various catalystic reactions.[3]
References
- Braude, E. A.; Nachod, F. C., eds. (1955). Determination of Organic Structure by Physical Methods. Academic Press. ISBN 9781483275727.
- Kuhn, Bernd; Mohr, Peter; Stahl, Martin (2010). "Intramolecular Hydrogen Bonding in Medicinal Chemistry". Journal of Medicinal Chemistry. 53 (6): 2601–2611. doi:10.1021/jm100087s. PMID 20175530.
- Goossen, Lukas J.; Rodríguez, Nuria; Melzer, Bettina; Linder, Christophe; Deng, Guojun; Levy, Laura M. (2007). "Biaryl Synthesis via Pd-Catalyzed Decarboxylative Coupling of Aromatic Carboxylates with Aryl Halides". Journal of the American Chemical Society. 129 (15): 4824–4833. doi:10.1021/ja068993+. PMID 17375927.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.