Ghost crab
Ghost crabs are semiterrestrial crabs of the subfamily Ocypodinae. They are common shore crabs in tropical and subtropical regions throughout the world, inhabiting deep burrows in the intertidal zone. They are generalist scavengers and predators of small animals. The name "ghost crab" derives from their nocturnality and their generally pale coloration.[1][2] They are also sometimes called sand crabs, though the name refers to various other crabs that do not belong to the subfamily.
| Ghost crabs | |
|---|---|
 | |
| Horned ghost crab (Ocypode ceratophthalma) in Krabi, Thailand | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Malacostraca |
| Order: | Decapoda |
| Suborder: | Pleocyemata |
| Infraorder: | Brachyura |
| Family: | Ocypodidae |
| Subfamily: | Ocypodinae Rafinesque, 1815 |
| Type genus | |
| Ocypode Weber, 1795 | |
| Genera | |
| |
Characteristics of the subfamily include one claw being larger than the other, thick and elongated eyestalks, and a box-like body. The differences in claw sizes, however, are not as marked as in male fiddler crabs. The subfamily includes 22 species in two genera.
Taxonomy
Ocypodinae is one of two subfamilies in the family Ocypodidae, the other being the fiddler crab subfamily, Ucinae. Both subfamilies have members in which one of the claw-bearing legs (the chelipeds) is much larger than the other. However, only male fiddler crabs exhibit this, while both male and female ghost crabs have unequally sized claws. The difference is also much more pronounced among fiddler crab males. The fiddler crabs' carapaces are broadened at the front, while the carapaces of ghost crabs are more or less box-like. Lastly, the eyes of ghost crabs have large and elongated eyestalks, with the corneas occupying the entire lower part, while in fiddler crabs the eyestalks are long and thin, with the corneas small and located at the tip.[3][4]
Ocypodinae was previously regarded as monotypic, with only one genus, Ocypode, classified under it. In 2013, though, Katsushi Sakai and Michael Türkay reclassified the gulf ghost crab into a separate genus, Hoplocypode based on the differences of their gonopods (appendages modified into copulatory organs) from that of the rest of the members of the genus Ocypode.[5]
Genera
The subfamily Ocypodinae currently contains 22 species in these two genera:[5]
- Ocypode Weber, 1795
- Hoplocypode Sakai &Türkay, 2013
Description

Most ghost crabs have pale-colored bodies that blend in well with the sand,[3] though they are capable of gradually changing body coloration to match their environments and the time of day.[6][7] Some species are brightly colored, such as Ocypode gaudichaudii and Ocypode ryderi.[2][5]
Ghost crabs have elongated and swollen eyestalks with very large corneas on the bottom half. Their carapaces are deep and box-like, squarish when viewed from the top with straight or slightly curving sides. The regions of the carapace are usually not clearly defined. The "whip" of their antennules (antennular flagella) are small or rudimentary. They fold back into the body diagonally or almost vertically. The plate between them (the interantennular septum) is broad. The third pair of mouth appendages (maxillipeds) completely cover the mouth opening. A small orifice with edges thickly fringed with hair is found between the bases of the second and third pairs of walking legs.[8]
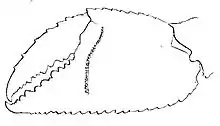
Ghost crab species can be most reliably identified by means of the area where they were recovered, the presence of "horns" (styles) on their eyestalks (exophthalmy), the pattern of stridulating (sound-producing) ridges on the inside surface of the palms of their larger claws, and the shape of the gonopods in males.[5]
Exophthalmy is exhibited by seven species in the subfamily: Ocypode brevicornis, Ocypode ceratophthalma, Ocypode gaudichaudii, Ocypode macrocera, Ocypode mortoni, Ocypode rotundata, and Ocypode saratan. All of them are found in the Indo-Pacific region with more or less limited ranges, with the exception of Ocypode ceratophthalma, which is found widely. Ocypode cursor, found in the Atlantic and the Mediterranean Sea, also possesses a tuft of bristles at the end of its eyestalks.[5]
Stridulating ridges also differ from species to species, with some displaying rows of tubercles, others displaying rows of smaller ridges (striae), or a combination of both. These are very important in identifying species as they are displayed by both adults and juveniles (though they may be absent in newly regenerated claws). They are used by ghost crabs for communication.[5]
Both exophthalmy and stridulating ridges, however, can not be reliably used to determine phylogenetic relationships between different species of ghost crabs. Sakai & Türkay (2013) regarded the shape of the gonopods as more suited for this. As gonopods are important in sexual reproduction, they are less likely to evolve randomly in response to the environment. Ghost crabs of the genus Hoplocypode can be distinguished from those in Ocypode by examining their gonopods. In the former, the first gonopod has a complex, hoof-shaped tip, while in the latter they are simple and curved.[5]
Ecology

Ghost crabs dig deep burrows near the intertidal zone of open sandy beaches. The burrows are usually composed of a long shaft with a chamber at the end, occasionally with a second entrance shaft.[1] They are semi-terrestrial and breathe oxygen from the air through moistened gills. They must periodically wet their gills with seawater,[1][9] usually by taking water from moist sand or by running into the surf and letting the waves wash over them. However, they can only remain under water for a limited amount of time, as they will drown.[10][11]
Ghost crabs are generalists, scavenging carrion and debris, as well as preying on small animals, including sea turtle eggs and hatchlings, clams, and other crabs.[12] They are predominantly nocturnal. They remain in their burrows during the hottest part of the day, and throughout the coldest part of the winter.[1]
Ghost crabs are swift runners, darting away at the slightest sign of danger. They either head back to their burrows or into the sea to escape intruders.[10][11][13] The gaits of ghost crabs alter as their speed increases. Observations on O. ceratophthalma show it can walk indefinitely using all four pairs of walking legs, occasionally alternating which side leads. At higher speeds, the fourth pair of legs is raised off the ground, and at the highest speeds, the crab runs, using only the first and second pairs of walking legs.[14]
Ghost crabs utilize a varied animal acoustic communication system. They can create different sounds by striking the ground with their claws, rubbing their claws together to make a rasping sound, rubbing their legs to make a bubbly noise, and rubbing the teeth inside their stomachs to make a growling sound. The lateral teeth of the gastric mill possess a series of comb-like structures that rub against the median tooth to produce stimulation with dominant frequencies below 2 kHz.[15]
Ghost crabs also have the ability to change colors to match their surroundings by adjusting the concentration and dispersal of pigments within their chromatophores.[6] They can even match the specific colors of the grains of sand in their habitats.[16] However, unlike metachrosis (which is a rapid change of colors), ghost crabs are only capable of morphological color change, which occurs over a longer span of time.[6]
.jpg.webp)
.jpg.webp) Horned ghost crab (Ocypode ceratophthalma) from a black sand beach in Kauai
Horned ghost crab (Ocypode ceratophthalma) from a black sand beach in Kauai
.jpg.webp)
In a study in 1964, individuals of O. ceratophthalma in Hawaii from two populations (one from a black-sand beach and the other from a white-sand beach) were observed to markedly differ in pigmentation although they remain morphologically indistinguishable. Specimens taken from the black-sand beach were much darker than the specimens taken from the white-sand beach, exhibiting nearly 12 times as many black chromatophores. When the black-sand specimens were subsequently exposed to a white background, they were observed to gradually become lighter over the course of about a month. The opposite happened to white-sand specimens when placed in black backgrounds.[6]

In a 2013 study in Singapore, Ocypode ceratophthalma was also discovered to change color in response to the time of day. In a span of 24 hours, they were observed to alternate between lighter and darker coloration, being lightest at midday and darkest at night. However, when placed in a dark background, the crabs showed no significant changes in coloration. This suggests, at least for short-term color changes, they follow a day-night cycle to determine body coloration; basing it on their biological clocks rather than on the brightness or darkness of their environments. The researchers believe relying on the time of day rather than ambient light is more advantageous for survival in ghost crabs. It lets ghost crabs avoid changing color when in temporary shadow (for example within their burrows) and thus still remain inconspicuous when they are once again illuminated by daylight. However, this behavior is only exhibited by juveniles.[7][16]
Ghost crabs lay their eggs in the sea, which develop into planktonic marine larvae.[1]
Distribution
Ghost crabs dominate sandy shores in tropical and subtropical regions of the world, replacing the sandhoppers that predominate in cooler areas.[1] Three species are found in the Atlantic Ocean and the Mediterranean Sea, and two occur in the eastern Pacific coast of the Americas. The rest of the species are found in the western Pacific and the Indian Ocean to the tip of southern Africa.[5]
Conservation
Ghost crabs are negatively affected by human activity on sandy beaches, such as sand trampling by foot traffic, the building of seawalls, or the presence of inorganic pollutants. Due to their worldwide distribution and the ease by which their burrows can be surveyed, ghost crab burrows are regarded as valuable ecological indicators for quickly assessing the impact of human disturbance on beach habitats.[20][21][22]
See also
- Heloecius - the semaphore crab
References
- George Karleskint; Richard Keith Turner; James Small (2009). "Intertidal communities". Introduction to Marine Biology (3rd ed.). Cengage Learning. pp. 356–411. ISBN 978-0-495-56197-2.
- Thomas G. Wolcott (1988). "Ecology". In Warren W. Burggren; Brian Robert McMahon (eds.). Biology of the land crabs. Cambridge University Press. pp. 55–97. ISBN 978-0-521-30690-4.
- Gary C. B. Poore; Shane T. Ahyong (2004). Marine Decapod Crustacea of Southern Australia: A Guide to Identification. CSIRO Publishing. p. 496. ISBN 9780643069060.
- S. Ajmal Khan; S. Ravichandran. "Brachyuran Crabs" (PDF). UNU-INWEH Course 1 - Training Course on Mangroves and Biodiversity, Institute for Water, Environment and Health, United Nations University. Retrieved November 7, 2013.
- Katsushi Sakai; Michael Türkay (2013). "Revision of the genus Ocypode with the description of a new genus, Hoplocypode (Crustacea: Decapoda: Brachyura)" (PDF). Memoirs of the Queensland Museum – Nature. 56 (2): 665–793.
- Jonathan P. Green (1964). "Morphological color change in the Hawaiian ghost crab Ocypode ceratophthalma (Pallas)" (PDF). The Biological Bulletin. 126 (3): 407–413. doi:10.2307/1539309. JSTOR 1539309.
- Martin Stevens; Cheo Pei Rong; Peter A. Todd (2013). "Colour change and camouflage in the horned ghost crab Ocypode ceratophthalmus". Biological Journal of the Linnean Society. 109 (2): 257–270. doi:10.1111/bij.12039.
- Katsushi Sakai & Masatsune Takeda( with contributions from Peter J.F. Davie, Danièle Guinot, and Michael Türkay). "Subfamily Ocypodinae". Crabs of Japan, Marine Species Identification Portal. Retrieved November 7, 2013.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Naturaliste Marine Discovery Centre. "Golden Ghost Crab". Government of Western Australia. Archived from the original on November 12, 2013. Retrieved November 12, 2013.
- K. G. Raja Bai Naidu (1951). "Some stages in the development and bionomics of Ocypoda platytarsis". Proceedings of the Indian Academy of Sciences, Section B. 33 (1): 32–40. doi:10.1007/BF03049973. S2CID 81531389.
- Smithsonian Marine Station's Field Guide to the Indian River Lagoon (2008–2011). "Ocypode quadrata". Smithsonian Marine Station at Fort Pierce. Retrieved November 12, 2013.
- Pat Garber (2006). "Phantoms in the Surf: Ghost Crabs". Ocracoke Wild: A Naturalist's Year on an Outer Banks Island. Parkway Publishers. pp. 94–98. ISBN 978-1-933251-31-8.
- Mary J. Rathbun (1921). "The brachyuran crabs collected by the American Museum Congo Expedition, 1909-1915". Bulletin of the American Museum of Natural History. 43: 379–474. hdl:2246/1016.
- C. F. Herreid II; R. J. Full (1988). "Energetics and locomotion". In Warren W. Burggren; Brian Robert McMahon (eds.). Biology of the land crabs. Cambridge University Press. pp. 333–377. ISBN 978-0-521-30690-4.
- Taylor JRA; Devries, M. S.; Elias, D. O. (2019). "JRA Taylor". Proceedings. Biological Sciences. 286 (1910). doi:10.1098/rspb.2019.1161. PMC 6742986. PMID 31506058.
- Ella Davies (April 5, 2013). "Horned ghost crabs change camouflage from day to night". BBC Nature. Retrieved November 8, 2013.
- Sabrina Trocini (2013). Health assessment and hatching success of two Western Australian loggerhead turtle (Caretta caretta) populations (PDF) (Ph.D.). Murdoch University.
- Brandon T. Barton (2009). Cascading effects of predator removal on the ecology of sea turtle nesting beaches (PDF) (Thesis). University of Idaho.
- Nick Atkinson (September 27, 2008). "Don't Tread On Me". Conservation, University of Washington. Retrieved November 14, 2013.
- Wagner Ferreira Magalhães; Juliana Barbosa Lima; Francisco Barros; José Maria Landim Dominguez (2009). "Is Ocypode quadrata (Fabricius, 1787) a useful tool for exposed sandy beaches management in Bahia State (Northeast Brazil)?". Brazilian Journal of Oceanography. 57 (2): 149–152. doi:10.1590/S1679-87592009000200008.
- Frederico Monteiro Neves; Carlos Emílio Bemvenuti (2006). "The ghost crab Ocypode quadrata (Fabricius, 1787) as a potential indicator of anthropic impact along the Rio Grande do Sul coast, Brazil". Biological Conservation. 133 (4): 431–435. doi:10.1016/j.biocon.2006.04.041.
- Denis Worlanyo Aheto; Cephas Asare; Emmanuel Abeashi Mensah; Joseph Aggrey-Fynn (2011). "Rapid assessment of anthropogenic impacts on exposed sandy beaches in Ghana using ghost crabs (Ocypode spp.) as ecological indicators". Momona Ethiopian Journal of Science. 3 (2): 93–103. doi:10.4314/mejs.v3i2.67715.
External links
- Taylor, Jennifer R. A., et al. “Growling from the Gut: Co-Option of the Gastric Mill for Acoustic Communication in Ghost Crabs.” Proceedings of the Royal Society B: Biological Sciences, vol. 286, no. 1910, 2019, p. 20191161., doi:10.1098/rspb.2019.1161.
- Martin Stevens, Alice Lown, Peter Todd & Cheo Pei Rong at the. "Camouflage and Colour Change in Ghost Crabs". Sensory Ecology & Evolution, University of Exeter. Archived from the original on 2015-09-19. Retrieved 2013-11-08.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Dennis DiClaudio (September 8, 2003). "The Nature of the Carolina Ghost Crab". Yankee Pot Roast.
- Video of Ocypode ceratophthalma eating a moon crab in Saint John's Island, Singapore
- Taylor, Jennifer R. A., et al. “Growling from the Gut: Co-Option of the Gastric Mill for Acoustic Communication in Ghost Crabs.” Proceedings of the Royal Society B: Biological Sciences, vol. 286, no. 1910, 2019, p. 20191161., doi:10.1098/rspb.2019.1161.