Ojima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima.[1] The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary.
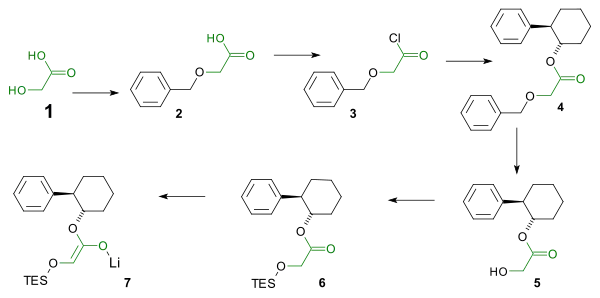
The reaction centers around an imine - lithium enolate cycloaddition. In order to ensure the correct stereochemistry (the phenyl group and the silyl ether must adopt a cis configuration) in the β-lactam a chiral auxiliary is used in the enolate synthesis. The enolate synthesis starts from glycolic acid. The hydroxyl group is protected by a benzyl group and the carboxylic acid is activated by reaction with thionyl chloride to the acid chloride. The acid chloride reacts with the chiral auxiliary trans-2-phenyl-1-cyclohexanol. The benzyl group is then removed and replaced by a TES silyl ether by reaction with triethylsilyl chloride. Reaction with phenyllithium affords the enolate.
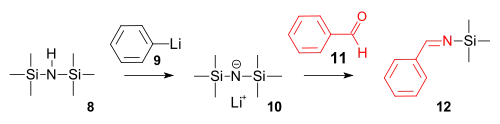
The imine synthesis is a reaction of hexamethyldisilazane with phenyllithium to a strong amide base followed by a condensation reaction with benzaldehyde.
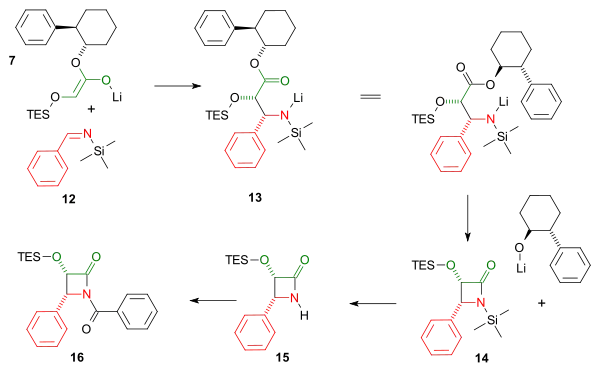
Both imine and enole intermediate join in a cycloaddition reaction followed by an intramolecular nucleophilic acyl substitution of the amine with expulsion of the chiral auxiliary to the cis-lactam. The triethylsilyl group is removed by hydrogen fluoride and the benzoyl group is added in a Schotten-Baumann reaction.
References
- Iwao Ojima; Ivan Habus; Mangzhu Zhao; Martine Zucco; Young Hoon Park; Chung Ming Sun; Thierry Brigaud (1992). "New and efficient approaches to the semisynthesis of taxol and its C-13 side chain analogs by means of lactam synthon method". Tetrahedron. 48 (34): 6985–7012. doi:10.1016/S0040-4020(01)91210-4.