Olutasidenib
Olutasidenib, sold under the brand name Rezlidhia, is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia.[1][2] Olutasidenib is an isocitrate dehydrogenase-1 (IDH1) inhibitor.[1] It is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Rezlidhia |
| Other names | FT-2102 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
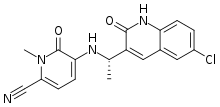
| Formula | C18H15ClN4O2 |
| Molar mass | 354.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The most common adverse reactions include nausea, fatigue/malaise, arthralgia, constipation, leukocytosis, dyspnea, fever, rash, mucositis, diarrhea, and transaminitis.[3]
Olutasidenib was approved for medical use in the United States in December 2022.[1][2][3][4][5]
Medical uses
Olutasidenib is indicated for the treatment of adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.[1][2][3]
Society and culture
Names
Olutasidenib is the international nonproprietary name.[6]
References
- "Rezlidhia- olutasidenib capsule". DailyMed. 13 December 2022. Retrieved 21 January 2023.
- https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215814Orig1s000ltr.pdf
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves olutasidenib for relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation". U.S. Food and Drug Administration (FDA). 1 December 2022. Retrieved 20 December 2022.
- "Rigel Announces U.S. FDA Approval of Rezlidhia (olutasidenib) for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia with a Susceptible IDH1 Mutation". Rigel Pharmaceuticals, Inc. (Press release). 1 December 2022. Retrieved 2 December 2022.
- "Rigel Announces U.S. FDA Approval of Rezlidhia (olutasidenib) for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia with a Susceptible IDH1 Mutation" (Press release). Rigel Pharmaceuticals. 1 December 2022. Retrieved 2 December 2022 – via PR Newswire.
- World Health Organization (2019). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82". WHO Drug Information. 33 (3). hdl:10665/330879.
Further reading
- Liu X, Gong Y (2019). "Isocitrate dehydrogenase inhibitors in acute myeloid leukemia". Biomarker Research. 7: 22. doi:10.1186/s40364-019-0173-z. PMC 6806510. PMID 31660152.
- Watts JM, Baer MR, Yang J, Prebet T, Lee S, Schiller GJ, et al. (November 2022). "Olutasidenib alone or with azacitidine in IDH1-mutated acute myeloid leukaemia and myelodysplastic syndrome: phase 1 results of a phase 1/2 trial". The Lancet Haematology. 10 (1): e46–e58. doi:10.1016/S2352-3026(22)00292-7. PMID 36370742. S2CID 253471380.
External links
- "Olutasidenib". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02719574 for "Open-label Study of FT-2102 With or Without Azacitidine or Cytarabine in Patients With AML or MDS With an IDH1 Mutation" at ClinicalTrials.gov