Organogermanium chemistry
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds.[1] Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.[2]
Synthesis
The great majority of organogermanium compounds are tetrahedral with the formula GeR4-nXn where X = H, Cl, etc. Ge-C bonds are air-stable, although Ge-H bonds can undergo air-oxidation. The first organogermanium compound, tetraethylgermane, synthesized by Winkler in 1887,[3] by the reaction of germanium tetrachloride with diethylzinc. More commonly, these Ge(IV) compounds are prepared by alkylation of germanium halides by organolithium and Grignard reagents. The method has been applied to surfaces terminated with Ge-Cl bonds.[4]
Some organogermanes are prepared by nucleophilic substitution or Pd-catalyzed cross-coupling reactions.[2] Hydrogermylation provides another route to organogermanium compounds.[5]

Catenation

Akin to hydrocarbons and polysilanes, many organogermanium compounds are known with Ge-Ge bonds. An early example is hexaphenyldigermane, (C6H5)3Ge−Ge(C6H5)3. It is prepared by Wurtz coupling of the bromide:[7]
- 2 (C6H5)3GeBr + 2 Na → (C6H5)3Ge−Ge(C6H5)3 + 2 NaBr
Many cyclic polygermanes are known, e.g. [Ge(C6H5)2]4, [Ge(C6H5)2]5, and [Ge(C6H5)2]6.
Multiple bonds to Ge
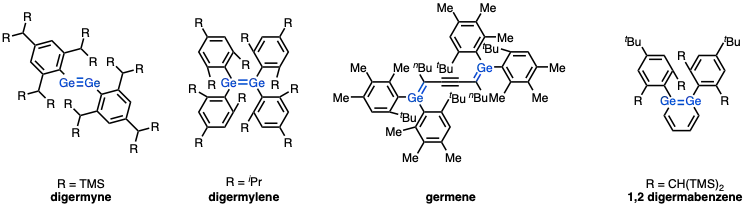
Compounds with multiple bonds to Ge are usually highly reactive or require bulky organic substituents for their isolation. This situation follows from the double bond rule. Digermynes only exist for extremely bulky substituents. According to X-ray crystallography, the C–Ge≡Ge–C core of digermynes is bent. Such compounds are prepared by the reduction of bulky arylgermanium(II) halides.[9]
Compounds containing Ge=C (germenes) double bonds require bulky organic substituents for their isolation.[10] and Ge=Ge (digermylenes)[11] Other examples include the bulky derivatives of germabenzene and 1,2-digermabenzene,[12] analogues of benzene.
Germylenes and germanium radicals
Germylenes (carbene analogues) and germyl free radicals have been investigated. Reaction of a Ge(II) chloride with a lithium trialkylgermanide affords a germylene:[13]
- ArGeCl + LiGe(C(CH3)3)3 → ArGeGe((C(CH3)3)3 + LiCl (Ar = 2,6-(mesityl)2C6H3)
Reactions of organogermanium compounds
Some organogermanium compounds participate in cross coupling reactions.
Applications
Organogermanium compounds are used in relatively few commercial applications. Isobutylgermane, a volatile colorless liquid, is used in MOVPE (Metalorganic Vapor Phase Epitaxy) in the deposition of Ge semiconductor films.
Propagermanium, also known as Ge-132, and spirogermanium are drugs.
References
- Yamamoto, Hisashi; Oshima, Koichiro (2004). Main Group Metals in Organic Synthesis. ISBN 3-527-30508-4.
- Fricke, Christoph; Schoenebeck, Franziska (2020-11-17). "Organogermanes as Orthogonal Coupling Partners in Synthesis and Catalysis". Accounts of Chemical Research. 53 (11): 2715–2725. doi:10.1021/acs.accounts.0c00527. ISSN 0001-4842. PMID 33118804. S2CID 226044725.
- Winkler, Clemens (1887-08-27). "Mittheilungen über das Germanium". Journal für Praktische Chemie. 36 (1): 177–209. doi:10.1002/prac.18870360119.
- Buriak, Jillian M. (2002). "Organometallic Chemistry on Silicon and Germanium Surfaces". Chemical Reviews. 102 (5): 1271–1308. doi:10.1021/cr000064s. PMID 11996538.
- Selmani, Aymane; Schoenebeck, Franziska (2023). "Anti-Markovnikov Hydrogermylation of Alkenes via Lewis Acid Catalysis". Synthesis. 55 (11): 1792–1798. doi:10.1055/a-2036-3868. ISSN 0039-7881. S2CID 256969453.
- Sekiguchi, Akira; Yatabe, Tetsuo; Kabuto, Chizuko; Sakurai, Hideki (1993). "Chemistry of organosilicon compounds. 303. The missing hexasilaprismane: Synthesis, x-ray analysis and photochemical reactions". Journal of the American Chemical Society. 115 (13): 5853–5854. doi:10.1021/ja00066a075.
- Amadoruge, Monika L.; Weinert, Charles S. (2008). "Singly Bonded Catenated Germanes: Eighty Years of Progress". Chemical Reviews. 108 (10): 4253–4294. doi:10.1021/cr800197r. PMID 18816144.
- Ferguson, G.; Gallagher, J. F.; Murphy, D.; Spalding, T. R.; Glidewell, C.; Holden, H. D. (1992-07-15). "The Structure of Triphenylgermanium Hydroxide". Acta Crystallographica Section C Crystal Structure Communications. 48 (7): 1228–1231. doi:10.1107/S0108270191015056.
- Sugiyama, Yusuke; Sasamori, Takahiro; Hosoi, Yoshinobu; Furukawa, Yukio; Takagi, Nozomi; Nagase, Shigeru; Tokitoh, Norihiro (2006-01-01). "Synthesis and Properties of a New Kinetically Stabilized Digermyne: New Insights for a Germanium Analogue of an Alkyne". Journal of the American Chemical Society. 128 (3): 1023–1031. doi:10.1021/ja057205y. ISSN 0002-7863. PMID 16417395.
- Meiners, Frank; Saak, Wolfgang; Weidenbruch, Manfred (2000-07-01). "Acetylene-Linked Bis(germaethenes): The First Molecules with Conjugated Germanium−Carbon Double Bonds 1". Organometallics. 19 (15): 2835–2836. doi:10.1021/om000284w. ISSN 0276-7333.
- Schäfer, Helmut; Saak, Wolfgang; Weidenbruch, Manfred (1999-08-01). "Azadigermiridines by Addition of Diazomethane or Trimethylsilyldiazomethane to a Digermene 1". Organometallics. 18 (16): 3159–3163. doi:10.1021/om990337d. ISSN 0276-7333.
- Sasamori, Takahiro; Sugahara, Tomohiro; Agou, Tomohiro; Guo, Jing-Dong; Nagase, Shigeru; Streubel, Rainer; Tokitoh, Norihiro (2015-06-08). "Synthesis and Characterization of a 1,2-Digermabenzene". Organometallics. 34 (11): 2106–2109. doi:10.1021/om501204u. ISSN 0276-7333.
- Setaka, Wataru; Sakamoto, Kenkichi; Kira, Mitsuo; Power, Philip P. (2001). "Synthesis and Structure of Stable Tri-tert-butylgermyl-Substituted Stannylene and Germylene". Organometallics. 20 (22): 4460–4462. doi:10.1021/om010591h.