Organophosphinic acid
An organophosphinic acid is an organophosphorus compound with the formula R2−nHnPO2H (R = alkyl, aryl). One or both P-H bonds in the parent hypophosphorous acid (aka phosphinic acid) are replaced by organic groups. The Cyanex family of dialkylphosphinic acids are used in hydrometallurgy to extract metals from ores.
Monalkylphosphinic acids
Monalkylphosphinic acids have the formula OP(OH)(H)R, with the simplest example being methylphosphinic acid.
Phosphinic acid adds to Michael acceptors, for example with acrylamide it gives H(HO)P(O)CH2CH2C(O)NH2.
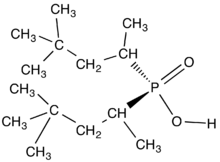
Dialkylphosphinic acids
Dialkylphosphinic acids have the formula R2PO2H, where R is an alkyl or aryl group. The phosphorus(V) center has tetrahedral molecular geometry. Under the brand names Aerophine and Cyanex, dialkylphosphinic acids are used in extraction and separation of metals as one of the techniques of hydrometallurgy[1] Characteristically the organic substituents are branched to confer solubility and preclude crystallization.[2]
Formaldehyde and H3PO2 react to give (HOCH2)2PO2H.
Related compounds
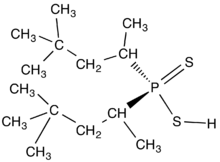
The dithiodialkyphosphinic acids (R2PS2H) are related to the diorganodithiophosphates with the formula (RO)2PS2H, which are also used as complexing agents in the purification of metals. The phosphates are more prone to hydrolysis owing to the greater lability of the RO-P linkage vs the direct C-P bond.
See also
- Phosphinate, salts of H2PO2−
References
- Svara, Jürgen; Weferling, Norbert; Hofmann, Thomas (2006). "Phosphorus Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_545.pub2.
- Fleitlikh, I.Yu.; Grigorieva, N.A.; Kuz'Min, V.I.; Pashkov, G.L. (2012). "Redox processes during cobalt extraction with bis(2,4,4-trimethylpentyl)dithiophosphinic acid". Hydrometallurgy. 129–130: 43–49. Bibcode:2012HydMe.129...43F. doi:10.1016/j.hydromet.2012.08.009.