Osladin
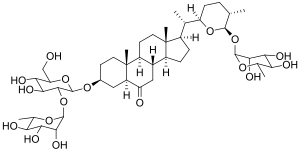
Osladine is a high-intensity sweetener isolated from the rhizome of Polypodium vulgare.[3] It is a saponin, sapogenin steroid glycoside, 500 times sweeter than sucrose.[4]
 | |
| Names | |
|---|---|
| IUPAC name
(22R,25S,26R)-26-(α-L-Rhamnopyranosyl)-3β-[α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosyloxy]-22,26-epoxy-5α-cholestan-6-one | |
| Systematic IUPAC name
(12S,13R,14R,15R,16S,32R,33R,34S,35S,36R,51R,53aS,53bS,55aS,57S,59aR,59bS,511aS,6S,72R,75S,76R,92S,93R,94R,95R,96S)-13,14,15,34,35,93,94,95-Octahydroxy-36-(hydroxymethyl)-16,59a,511a,6,75,96-hexamethylhexadecahydro-55H-2,4,8-trioxa-1,9(2),3(3,2),7(2,6)-tetrakis(oxana)-5(7,1)-cyclopenta[a]phenanthrenanonaphan-55-one | |
| Other names
26-O-α-L-Rhamnopyranosyl-(22R,25S,26R)-22,26-epoxy-6-oxo-5α-cholestan-3β,26-diol-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C45H74O17 | |
| Molar mass | 887.070 g·mol−1 |
| Appearance | White crystals[1] |
| Melting point | 202 to 204 °C (396 to 399 °F; 475 to 477 K)[1] |
| Low in water.[2] Soluble in ethanol.[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
A related compound, polypodoside A, has been identified from Polypodium glycyrrhiza and is 600 times sweeter than a sucrose solution at 6%.[2]
See also
References
- C.-R. Yang & O. Tanaka: Advances in Plant Glycosides, Chemistry and Biology. in Proceedings of the International Symposium on Plant Glycosides, August 12-15, 1997, Kunming, China; Elsevier, 1999. ISBN 978-0-444-50180-6
- AD Kinghorn & CM Compadre, Alternative Sweeteners: Third Edition, Revised and Expanded, Marcel Dekker, New York, 2001. ISBN 0-8247-0437-1
- J Jizba, L Dolejs, V Herout & F Sorm, « The structure of osladin — The sweet principle of the rhizomes of Polypodium vulgare L. », dans Tetrahedron Lett., vol. 18, 1971, p. 1329-1332 doi:10.1016/S0040-4039(01)96701-2
- Yamada, H. und Nishizawa, M. (1995): Synthesis and Structure Revision of Intensely Sweet Saponin Osladin. In: J Org Chem. 60(2); 386–397; doi:10.1021/jo00107a018
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.