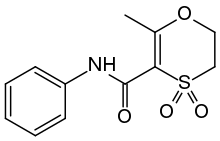
Oxycarboxin
Oxycarboxin is an organic chemical used in agriculture to protect crops from fungal diseases. It was first marketed by Uniroyal in 1969 using their brand name Plantvax. The compound is an anilide which combines a heterocyclic acid with aniline to give an inhibitor of succinate dehydrogenase (SDHI).[1][2][3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
6-Methyl-4,4-dioxo-N-phenyl-3,4-dihydro-2H-1,4λ6-oxathiine-5-carboxamide | |
| Other names
Oxycarboxine; Dcmod; Oxicarboxin, Vitavax sulfone, Plantvax, Carbojet, 5,6-dihydro-2-methyl-1,4-oxathi-ine-3-carboxanilide-4,4-dioxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.023.697 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H13NO4S | |
| Molar mass | 267.30 g·mol−1 |
| Melting point | 120 °C (248 °F; 393 K) |
| moderate | |
| Solubility | acetone, DMF, ethanol, and methanol |
| Related compounds | |
Related compounds |
Carboxin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
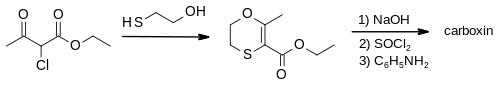
Oxyxarboxin was first made by the oxidation of carboxin, as disclosed in patents filed by Uniroyal.[4]
Ethyl 2-chloroacetoacetate is treated with 2-mercaptoethanol and base, followed by cyclisation and water removal under acidic conditions. The resultant ethyl ester of the 1,4-oxathiine heterocycle is then formed into an amide with aniline using standard conditions via the carboxylic acid and acid chloride. This gives carboxin in high overall yield.[5] The synthesis is completed by treatment with 30% hydrogen peroxide in acetic acid.[4]
Mechanism of action
Carboxin and oxycarboxin act by inhibition of succinate dehydrogenase (SDHI):[6] they bind to the quinone reduction site of the enzyme complex, preventing ubiquinone from doing so. As a consequence, the tricarboxylic acid cycle and electron transport chain cannot function.[7][8]
Uses
Oxycarboxin is used to control rust diseases (e.g. soybean rust) at an application rate of 200–400 g/ha.[3][9]
History
Oxycarboxin has been commercially available since 1969, when it was introduced under the brand name Plantvax.[2][10]
References
- Walter, Harald (2016). "Fungicidal Succinate-Dehydrogenase-Inhibiting Carboxamides". In Lamberth, Clemens; Dinges, Jürgen (eds.). Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals. Wiley. pp. 405–425. doi:10.1002/9783527693931.ch31. ISBN 9783527339471.
- Ackermann, Peter; Margot, Paul; Müller, Franz (2000). "Fungicides, Agricultural". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a12_085. ISBN 3527306730.
- Shanmugasundaram, S.; Yeh, C.C.; Hartman, G.L.; Talekar, N.S. (1991). Vegetable Soybean Research Needs for Production and Quality Improvement (PDF). Taipei: Asian Vegetable Research and Development Center. pp. 86–87. ISBN 9789290580478. Retrieved 6 February 2016.
- US patent 3402241, von Schmeling, B.; Kulka, M. & Harrison, W.A., "Control of plant fungal and bacterial diseases with 2, 3-dihydro-5-carboximido-6-methyl-1, 4-oxathiin, mono-and di-oxides", issued 1968-09-17, assigned to Uniroyal Inc
- von Schmeling, B.; Kulka, Marshall (1966). "Systemic Fungicidal Activity of 1,4-Oxathiin Derivatives". Science. 152 (3722): 659–660. Bibcode:1966Sci...152..659V. doi:10.1126/science.152.3722.659. PMID 17779512. S2CID 27561137.
- Gunatilleke, I. A. U. N.; Arst, H. N.; Scazzocchio, C. (1975). "Three genes determine the carboxin sensitivity of mitochondrial succinate oxidation in Aspergillus nidulans". Genetical Research. 26 (3): 297–305. doi:10.1017/S0016672300016098. PMID 178574.
- Oyedotun, Kayode S.; Lemire, Bernard D. (2004). "The Quaternary Structure of the Saccharomyces cerevisiae Succinate Dehydrogenase". Journal of Biological Chemistry. 279 (10): 9424–9431. doi:10.1074/jbc.M311876200. PMID 14672929.
- Avenot, Hervé F.; Michailides, Themis J. (2010). "Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi". Crop Protection. 29 (7): 643–651. doi:10.1016/j.cropro.2010.02.019.
- Worthing C.R., ed. (1987). The Pesticide Manual - A World Compendium (Eighth ed.). British Crop Protection Council. p. 624. ISBN 0948404019.
- Pesticide Properties Database (2023-06-15). "Oxycarboxin". University of Hertfordshire. Retrieved 2023-08-07.