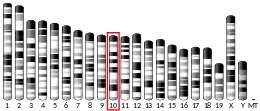
P4HA1
Prolyl 4-hydroxylase subunit alpha-1 is an enzyme that in humans is encoded by the P4HA1 gene.[5][6]
This gene encodes a component of prolyl 4-hydroxylase, a key enzyme in collagen synthesis composed of two identical alpha subunits and two beta subunits. The encoded protein is one of several different types of alpha subunits and provides the major part of the catalytic site of the active enzyme. In collagen and related proteins, prolyl 4-hydroxylase catalyzes the formation of 4-hydroxyproline that is essential to the proper three-dimensional folding of newly synthesized procollagen chains. Alternatively spliced transcript variants encoding different isoforms have been described.[6]
References
- GRCh38: Ensembl release 89: ENSG00000122884 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000019916 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Pajunen L, Jones TA, Helaakoski T, Pihlajaniemi T, Solomon E, Sheer D, Kivirikko KI (Jan 1990). "Assignment of the gene coding for the alpha-subunit of prolyl 4-hydroxylase to human chromosome region 10q21.3-23.1". Am J Hum Genet. 45 (6): 829–34. PMC 1683466. PMID 2556027.
- "Entrez Gene: P4HA1 procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), alpha polypeptide I".
Further reading
- Helaakoski T, Vuori K, Myllylä R, et al. (1989). "Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts". Proc. Natl. Acad. Sci. U.S.A. 86 (12): 4392–6. doi:10.1073/pnas.86.12.4392. PMC 287275. PMID 2543975.
- Helaakoski T, Veijola J, Vuori K, et al. (1994). "Structure and expression of the human gene for the alpha subunit of prolyl 4-hydroxylase. The two alternatively spliced types of mRNA correspond to two homologous exons the sequences of which are expressed in a variety of tissues". J. Biol. Chem. 269 (45): 27847–54. doi:10.1016/S0021-9258(18)46864-0. PMID 7961714.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Annunen P, Helaakoski T, Myllyharju J, et al. (1997). "Cloning of the human prolyl 4-hydroxylase alpha subunit isoform alpha(II) and characterization of the type II enzyme tetramer. The alpha(I) and alpha(II) subunits do not form a mixed alpha(I)alpha(II)beta2 tetramer". J. Biol. Chem. 272 (28): 17342–8. doi:10.1074/jbc.272.28.17342. PMID 9211872.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Horelli-Kuitunen N, Kvist AP, Helaakoski T, et al. (1998). "The order and transcriptional orientation of the human COL13A1 and P4HA genes on chromosome 10 long arm determined by high-resolution FISH". Genomics. 46 (2): 299–302. doi:10.1006/geno.1997.5015. PMID 9417920.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Deloukas P, Earthrowl ME, Grafham DV, et al. (2004). "The DNA sequence and comparative analysis of human chromosome 10". Nature. 429 (6990): 375–81. Bibcode:2004Natur.429..375D. doi:10.1038/nature02462. PMID 15164054.
- Raveendran M, Senthil D, Utama B, et al. (2004). "Cigarette suppresses the expression of P4Halpha and vascular collagen production". Biochem. Biophys. Res. Commun. 323 (2): 592–8. doi:10.1016/j.bbrc.2004.08.129. PMID 15369792.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Fähling M, Mrowka R, Steege A, et al. (2006). "Heterogeneous nuclear ribonucleoprotein-A2/B1 modulate collagen prolyl 4-hydroxylase, alpha (I) mRNA stability". J. Biol. Chem. 281 (14): 9279–86. doi:10.1074/jbc.M510925200. PMID 16464861.
- Chen L, Shen YH, Wang X, et al. (2006). "Human prolyl-4-hydroxylase alpha(I) transcription is mediated by upstream stimulatory factors". J. Biol. Chem. 281 (16): 10849–55. doi:10.1074/jbc.M511237200. PMC 2819823. PMID 16488890.
- Fähling M, Mrowka R, Steege A, et al. (2006). "Translational control of collagen prolyl 4-hydroxylase-alpha(I) gene expression under hypoxia". J. Biol. Chem. 281 (36): 26089–101. doi:10.1074/jbc.M604939200. PMID 16837461.
- Grimmer C, Balbus N, Lang U, et al. (2006). "Regulation of type II collagen synthesis during osteoarthritis by prolyl-4-hydroxylases: possible influence of low oxygen levels". Am. J. Pathol. 169 (2): 491–502. doi:10.2353/ajpath.2006.050738. PMC 1698781. PMID 16877351.
- Koivunen P, Hirsilä M, Kivirikko KI, Myllyharju J (2006). "The length of peptide substrates has a marked effect on hydroxylation by the hypoxia-inducible factor prolyl 4-hydroxylases". J. Biol. Chem. 281 (39): 28712–20. doi:10.1074/jbc.M604628200. PMID 16885164.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.