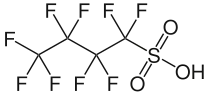
Perfluorobutanesulfonic acid
Perfluorobutanesulfonic acid (PFBS) is a PFAS chemical compound having a four-carbon fluorocarbon chain and a sulfonic acid functional group. It is stable and unreactive because of the strength of carbon–fluorine bonds. It can occur in the form of a colorless liquid or a corrosive solid.[3] Its conjugate base is perfluorobutanesulfonate (also called nonaflate) which functions as the hydrophobe in fluorosurfactants.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulfonic acid | |
| Other names
FC-98 Nonaflate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.176 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3094, 3265 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4HF9O3S | |
| Molar mass | 300.10 g/mol |
| Boiling point | 211 °C (412 °F; 484 K)[1] |
| Hazards | |
| GHS labelling: | |
  [2] [2] | |
| Danger | |
| H302, H314 | |
| P280, P305+P351+P338, P310[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Since June 2003, 3M has used PFBS as a replacement for the persistent, toxic, and bioaccumulative compound perfluorooctanesulfonic acid (PFOS) in its Scotchgard stain repellents.[4] 3M markets surfactant with PFBS in two fluorosurfactants.[5]
Safety
PFBS has a half-life of a little over one month in humans, much shorter than PFOS with 5.4 years.[6] PFBS is persistent in the environment. Studies have not yet been specifically conducted to determine safety in humans.
The ECHA decision adding PFBS and its salts to the REACH Regulation Candidate List of Substances of Very High Concern states:
"The combined intrinsic properties justifying the inclusion as a substance for which there is scientific evidence of probable serious effects to human health and the environment which give rise to an equivalent level of concern are the following: very high persistence, high mobility in water and soil, high potential for long-range transport, and difficulty of remediation and water purification as well as moderate bioaccumulation in humans. The observed probable serious effects for human health and the environment are thyroid hormonal disturbances and reproductive toxicity seen in rodents, and effects on liver, kidney and haematological system in rats, hormonal disturbances and effects on reproduction in marine medaka fish and effects on expression of hormone receptors in tadpoles. Together, these elements lead to a very high potential for irreversible effects."[7]
Legislation and regulation
Canada
British Columbia currently provides soil standards for perfluorobutane sulfonate (PFBS).[8] In November 2017, the BC Ministry of Environment and Climate Change Strategy released soil and water standards for three PFAS including perfluorobutane sulfonate to the British Columbia Contaminated Sites Regulation.[9]
European Union
On 2020-01-16, PFBS and its salts were added to the REACH Regulation Candidate List of Substances of Very High Concern (SVHCs) on the grounds of "Equivalent level of concern having probable serious effects to human health (Article 57(f) – human health)" and "Equivalent level of concern having probable serious effects to the environment (Article 57(f) – environment)".[7]
United States
In 2023, the EPA proposed a drinking water standard that included PFBS.[10] A few states have proposed or implemented regulations on PFBS in drinking watering either as contamination standards, guidance or health advisories.[11]
In 2020, Michigan adopted drinking water standards for 5 previously unregulated PFAS compounds, including PFBS which has a maximum contaminant level (MCL) of 420 parts per trillion (ppt).[12][13]
See also
References
- Perfluorobutanesulfonic acid in the ChemIDplus database
- Sigma-Aldrich Co., Nonafluorobutane-1-sulfonic acid. Retrieved on 15 January 2018.
- "Perfluorobutanesulfonic acid". PubChem. NIH. Retrieved 10 August 2021.
- Ullah, Aziz (October 2006). "The Fluorochemical Dilemma: What the PFOS/PFOA fuss is all about" (PDF). Cleaning & Restoration. Retrieved 16 January 2009.
- Renner R (January 2006). "The long and the short of perfluorinated replacements". Environ. Sci. Technol. 40 (1): 12–3. doi:10.1021/es062612a. PMID 16433328.
- Betts KS (May 2007). "Perfluoroalkyl acids: what is the evidence telling us?". Environ. Health Perspect. 115 (5): A250–6. doi:10.1289/ehp.115-a250. PMC 1867999. PMID 17520044.
- Schilliger-Musset, Christel (2020-01-16). "Inclusion of substances of very high concern in the Candidate List for eventual inclusion in Annex XIV" (PDF). European Chemicals Agency (ECHA).
- "PFAS in Canadian Provinces: Where are the environmental regulations?". Environmental Journal. March 27, 2023. Retrieved 9 July 2023.
- SLR Consulting (May 2019). "Guidance for the Assessment and Remediation of Per- and Polyfluoroalkyl Substances in British Columbia" (PDF). Society of Contaminated Sites Approved Professionals of British Columbia. Retrieved 9 July 2023.
- "Proposed PFAS National Primary Drinking Water Regulation". EPA. Retrieved 9 July 2023.
- "State-by-State Regulation of PFAS Substances in Drinking Water". Bryan Cave Leighton Paisner LLP. January 22, 2021. Retrieved 11 August 2021.
- Matheny, Keith (3 August 2020). "Michigan's drinking water standards for these chemicals now among toughest in nation". Detroit Free Press. Archived from the original on 31 January 2022. Retrieved 31 March 2022.
- "New state drinking water standards pave way for expansion of Michigan's PFAS clean-up efforts". Michigan.gov. 3 August 2020. Archived from the original on 3 January 2022. Retrieved 5 April 2022.