PINK1
PTEN-induced kinase 1 (PINK1) is a mitochondrial serine/threonine-protein kinase encoded by the PINK1 gene.[5][6]
It is thought to protect cells from stress-induced mitochondrial dysfunction. PINK1 activity causes the parkin protein to bind to depolarized mitochondria to induce autophagy of those mitochondria.[7][8] PINK1 is processed by healthy mitochondria and released to trigger neuron differentiation.[9] Mutations in this gene cause one form of autosomal recessive early-onset Parkinson's disease.[10]
Structure
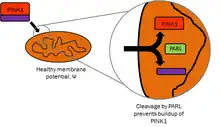
PINK1 is synthesized as a 63000 Da protein which is often cleaved by PARL, between the 103-Alanine and the 104-Phenylalanine residues, into a 53000 Da fragment.[11] PINK1 contains an N-terminal mitochondrial localization sequence, a putative transmembrane sequence, a Ser/Thr kinase domain, and a C-terminal regulatory sequence. The protein has been found to localize to the outer membrane of mitochondria, but can also be found throughout the cytosol. Experiments suggest the Ser/Thr kinase domain faces outward toward the cytosol, indicating a possible point of interaction with parkin.[12]
The structure of PINK1 has been solved and shows how the protein binds and phosphorylates its substrate ubiquitin.[13]
Function
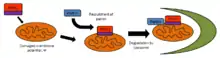
PINK1 is intimately involved with mitochondrial quality control by identifying damaged mitochondria and targeting specific mitochondria for degradation. Healthy mitochondria maintain a membrane potential that can be used to import PINK1 into the inner membrane where it is cleaved by PARL and cleared from the outer membrane. Severely damaged mitochondria lack sufficient membrane potential to import PINK1, which then accumulates on the outer membrane. PINK1 then recruits parkin to target the damaged mitochondria for degradation through autophagy.[14] Due to the presence of PINK1 throughout the cytoplasm, it has been suggested that PINK1 functions as a "scout" to probe for damaged mitochondria.[15]
PINK1 may also control mitochondria quality through mitochondrial fission. Through mitochondrial fission, a number of daughter mitochondria are created, often with an uneven distribution in membrane potential. Mitochondria with a strong, healthy membrane potential were more likely to undergo fusion than mitochondria with low membrane potential. Interference with the mitochondrial fission pathway led to an increase in oxidized proteins and a decrease in respiration.[16] Without PINK1, parkin cannot efficiently localize to damaged mitochondria, while an over-expression of PINK1 causes parkin to localize to even healthy mitochondria.[17] Furthermore, mutations in both Drp1, a mitochondrial fission factor, and PINK1 were fatal in Drosophila models. However, an over-expression of Drp1 could rescue subjects deficient in PINK1 or parkin, suggesting mitochondrial fission initiated by Drp1 recreates the same effects of the PINK1/parkin pathway.[18]
In addition to mitochondrial fission, PINK1 has been implicated in mitochondrial motility. The accumulation of PINK1 and recruitment of parkin targets a mitochondrion for degradation, and PINK1 may serve to enhance degradation rates by arresting mitochondrial motility. Over-expression of PINK1 produced similar effects to silencing Miro, a protein closely associated with mitochondrial migration.[19]
Another mechanism of mitochondrial quality control may arise through mitochondria-derived vesicles. Oxidative stress in mitochondria can produce potentially harmful compounds including improperly folded proteins or reactive oxygen species. PINK1 has been shown to facilitate the creation of mitochondria-derived vesicles which can separate reactive oxygen species and shuttle them toward lysosomes for degradation.[20]
Disease relevance
Parkinson's disease is often characterized by the degeneration of dopaminergic neurons and associated with the build-up of improperly folded proteins and Lewy bodies. Mutations in the PINK1 protein have been shown to lead to a build-up of such improperly folded proteins in the mitochondria of both fly and human cells.[21] Specifically, mutations in the serine/threonine kinase domain have been found in a number of Parkinson's patients where PINK1 fails to protect against stress-induced mitochondrial dysfunction and apoptosis.[22]
Pharmacological manipulation
To date, there have been few reports of small molecules that activate PINK1 and their promise as potential treatments for Parkinson's disease. The first report appeared in 2013 when Kevan Shokat and his team from UCSF identified a nucleobase called kinetin as an activator of PINK1.[23] Subsequently, it was shown by others that the nucleoside derivative of kinetin, i.e. kinetin riboside, exhibited significant activation of PINK1 in cells.[24] Additionally, the monophosphate prodrugs of kinetin riboside, ProTides, also showed activation of PINK1.[25] In December 2017, niclosamide, an anthelmintic drug, was identified as a potent activator of PINK1 in cells and in neurons.[26]
References
- GRCh38: Ensembl release 89: ENSG00000158828 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028756 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Unoki M, Nakamura Y (Aug 2001). "Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway". Oncogene. 20 (33): 4457–65. doi:10.1038/sj.onc.1204608. PMID 11494141.
- Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio AR (Sep 2004). "PINK1 mutations are associated with sporadic early-onset parkinsonism". Ann Neurol. 56 (3): 336–41. doi:10.1002/ana.20256. PMID 15349860. S2CID 11049051.
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ (2010). "PINK1 is selectively stabilized on impaired mitochondria to activate Parkin". PLOS Biology. 8 (1): e1000298. doi:10.1371/journal.pbio.1000298. PMC 2811155. PMID 20126261.
- Lazarou M, Narendra DP, Jin SM, Tekle E, Banerjee S, Youle RJ (2013). "PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding". Journal of Cell Biology. 200 (2): 163–172. doi:10.1083/jcb.201210111. PMC 3549971. PMID 23319602.
- Dagda RK, Pien I, Wang R, Zhu J, Wang KZ, Callio J, Banerjee TD, Dagda RY, Chu CT (2013). "Beyond the mitochondrion: cytosolic PINK1 remodels dendrites through protein kinase A". J Neurochem. 128 (6): 864–877. doi:10.1111/jnc.12494. PMC 3951661. PMID 24151868.
- "Entrez Gene: PINK1 PTEN induced putative kinase 1".
- Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SH, Renton AE, Harvey RJ, Whitworth AJ, Martins LM, Abramov AY, Wood NW (2011). "PINK1 cleavage at position A103 by the mitochondrial protease PARL". Hum. Mol. Genet. 20 (5): 867–869. doi:10.1093/hmg/ddq526. PMC 3033179. PMID 21138942.
- Springer W, Kahle PJ (March 2011). "Regulation of PINK1-Parkin-mediated mitophagy". Autophagy. 7 (3): 266–78. doi:10.4161/auto.7.3.14348. PMID 21187721.
- Schubert, Alexander F.; Gladkova, Christina; Pardon, Els; Wagstaff, Jane L.; Freund, Stefan M. V.; Steyaert, Jan; Maslen, Sarah L.; Komander, David (2017-10-30). "Structure of PINK1 in complex with its substrate ubiquitin". Nature. 552 (7683): 51–56. Bibcode:2017Natur.552...51S. doi:10.1038/nature24645. ISSN 1476-4687. PMC 6020998. PMID 29160309.
- Youle RJ, van der Bliek AM (2012). "Mitochondrial fission, fusion, and stress". Science. 337 (6098): 1062–1065. Bibcode:2012Sci...337.1062Y. doi:10.1126/science.1219855. PMC 4762028. PMID 22936770.
- Narendra D, Walker JE, Youle R (2012). "Mitochondrail quality control mediated by PINK1 and Parkin: links to parkinsonism". Cold Spring Harbor Perspectives in Biology. 4 (11): a011338. doi:10.1101/cshperspect.a011338. PMC 3536340. PMID 23125018.
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS (2008). "Fission and selective fusion govern mitochondrial segregation and elimination by autophagy". The EMBO Journal. 27 (2): 433–446. doi:10.1038/sj.emboj.7601963. PMC 2234339. PMID 18200046.
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrané J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S (2010). "PINK1-dependent recruitment of Parkin to mitochondria in mitophagy". Proceedings of the National Academy of Sciences of the United States of America. 107 (1): 378–83. Bibcode:2010PNAS..107..378V. doi:10.1073/pnas.0911187107. PMC 2806779. PMID 19966284.
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ (2008). "The PINK1/Parkin pathway regulates mitochondrial mitophagy". Proceedings of the National Academy of Sciences of the United States of America. 105 (5): 1638–43. doi:10.1073/pnas.0709336105. PMC 2234197. PMID 18230723.
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B (2012). "Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria". PLOS Genetics. 8 (3): e102537. doi:10.1371/journal.pgen.1002537. PMC 3291531. PMID 22396657.
- McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA (2014). "Parkin and PINK 1 function in a vesicular trafficking pathway regulating mitochondrial quality control". The EMBO Journal. 33 (4): 282–295. doi:10.1002/embj.201385902. PMC 3989637. PMID 24446486.
- Pimenta de Castro I, Costa AC, Lam D, Tufi R, Fedele V, Moisoi N, Dinsdale D, Deas E, Loh SH, Martins LM (2012). "Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster". Cell Death & Differentiation. 19 (8): 1308–16. doi:10.1038/cdd.2012.5. PMC 3392634. PMID 22301916.
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (2004). "Hereditary early-onset Parkinson's disease caused by mutations in PINK1". Science. 304 (5674): 1158–60. Bibcode:2004Sci...304.1158V. doi:10.1126/science.1096284. PMID 15087508. S2CID 33630092.
- Hertz NT, Berthet A, Sos ML, Thorn KS, Burlingame AL, Nakamura K, Shokat KM (2013). "A neo-substrate that amplifies catalytic activity of parkinson's-disease-related kinase PINK1". Cell. 154 (4): 737–47. doi:10.1016/j.cell.2013.07.030. PMC 3950538. PMID 23953109.
- Osgerby L, Lai YC, Thornton PJ, Amalfitano J, Le Duff CS, Jabeen I, Kadri H, Miccoli A, Tucker JH, Muqit M, Mehellou Y (2017). "Kinetin Riboside and Its ProTides Activate the Parkinson's Disease Associated PTEN-Induced Putative Kinase 1 (PINK1) Independent of Mitochondrial Depolarization". J. Med. Chem. 60 (8): 3518–24. doi:10.1021/acs.jmedchem.6b01897. PMC 5410652. PMID 28323427.
- Osgerby L, Lai YC, Thornton PJ, Amalfitano J, Le Duff CS, Jabeen I, Kadri H, Miccoli A, Tucker JH, Muqit M, Mehellou Y (2017). "Kinetin Riboside and Its ProTides Activate the Parkinson's Disease Associated PTEN-Induced Putative Kinase 1 (PINK1) Independent of Mitochondrial Depolarization". J. Med. Chem. 60 (8): 3518–24. doi:10.1021/acs.jmedchem.6b01897. PMC 5410652. PMID 28323427.
- Barini E, Miccoli A, Tinarelli F, Mulholand K, Kadri H, Khanim F, Stojanovski L, Read KD, Burness K, Blow JJ, Mehellou Y, Muqit M (2017). "The Anthelmintic Drug Niclosamide and its Analogues Activate the Parkinson's Disease Associated Protein Kinase PINK1". ChemBioChem. 19 (5): 425–429. doi:10.1002/cbic.201700500. PMC 5901409. PMID 29226533.
Further reading
- Heutink P (2006). "PINK-1 and DJ-1 — new genes for autosomal recessive Parkinson's disease". Parkinson's Disease and Related Disorders. pp. 215–9. doi:10.1007/978-3-211-45295-0_33. ISBN 978-3-211-28927-3. PMID 17017532.
{{cite book}}:|journal=ignored (help) - Valente EM, Bentivoglio AR, Dixon PH, Ferraris A, Ialongo T, Frontali M, Albanese A, Wood NW (2001). "Localization of a Novel Locus for Autosomal Recessive Early-Onset Parkinsonism, PARK6, on Human Chromosome 1p35-p36". Am. J. Hum. Genet. 68 (4): 895–900. doi:10.1086/319522. PMC 1275643. PMID 11254447.
- Khan NL, Valente EM, Bentivoglio AR, Wood NW, Albanese A, Brooks DJ, Piccini P (2002). "Clinical and subclinical dopaminergic dysfunction in PARK6-linked parkinsonism: an 18F-dopa PET study". Ann. Neurol. 52 (6): 849–53. doi:10.1002/ana.10417. PMID 12447943. S2CID 9275470.
- Bonifati V, Dekker MC, Vanacore N, Fabbrini G, Squitieri F, Marconi R, Antonini A, Brustenghi P, Dalla Libera A, De Mari M, Stocchi F, Montagna P, Gallai V, Rizzu P, van Swieten JC, Oostra B, van Duijn CM, Meco G, Heutink P (2003). "Autosomal recessive early onset parkinsonism is linked to three loci: PARK2, PARK6, and PARK7". Neurol. Sci. 23 (Suppl 2): S59–60. doi:10.1007/s100720200069. PMID 12548343. S2CID 13625056.
- Valente EM, Brancati F, Caputo V, Graham EA, Davis MB, Ferraris A, Breteler MM, Gasser T, Bonifati V, Bentivoglio AR, De Michele G, Dürr A, Cortelli P, Filla A, Meco G, Oostra BA, Brice A, Albanese A, Dallapiccola B, Wood NW (2003). "PARK6 is a common cause of familial parkinsonism". Neurol. Sci. 23 (Suppl 2): S117–8. doi:10.1007/s100720200097. PMID 12548371. S2CID 21061495.
- Nakajima A, Kataoka K, Hong M, Sakaguchi M, Huh NH (2004). "BRPK, a novel protein kinase showing increased expression in mouse cancer cell lines with higher metastatic potential". Cancer Lett. 201 (2): 195–201. doi:10.1016/S0304-3835(03)00443-9. PMID 14607334.
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (2004). "Hereditary early-onset Parkinson's disease caused by mutations in PINK1". Science. 304 (5674): 1158–60. Bibcode:2004Sci...304.1158V. doi:10.1126/science.1096284. PMID 15087508. S2CID 33630092.
- Healy DG, Abou-Sleiman PM, Ahmadi KR, Muqit MM, Bhatia KP, Quinn NP, Lees AJ, Latchmann DS, Goldstein DB, Wood NW (2004). "The gene responsible for PARK6 Parkinson's disease, PINK1, does not influence common forms of parkinsonism". Ann. Neurol. 56 (3): 329–35. doi:10.1002/ana.20206. PMID 15349859. S2CID 1235813.
- Hatano Y, Li Y, Sato K, Asakawa S, Yamamura Y, Tomiyama H, Yoshino H, Asahina M, Kobayashi S, Hassin-Baer S, Lu CS, Ng AR, Rosales RL, Shimizu N, Toda T, Mizuno Y, Hattori N (2004). "Novel PINK1 mutations in early-onset parkinsonism". Ann. Neurol. 56 (3): 424–7. doi:10.1002/ana.20251. PMID 15349870. S2CID 10853835.
- Hatano Y, Sato K, Elibol B, Yoshino H, Yamamura Y, Bonifati V, Shinotoh H, Asahina M, Kobayashi S, Ng AR, Rosales RL, Hassin-Baer S, Shinar Y, Lu CS, Chang HC, Wu-Chou YH, Ataç FB, Kobayashi T, Toda T, Mizuno Y, Hattori N (2004). "PARK6-linked autosomal recessive early-onset parkinsonism in Asian populations". Neurology. 63 (8): 1482–5. doi:10.1212/01.wnl.0000142258.29304.fe. PMID 15505170. S2CID 13480500.
- Healy DG, Abou-Sleiman PM, Gibson JM, Ross OA, Jain S, Gandhi S, Gosal D, Muqit MM, Wood NW, Lynch T (2006). "PINK1 (PARK6) associated Parkinson disease in Ireland". Neurology. 63 (8): 1486–8. doi:10.1212/01.wnl.0000142089.38301.8e. PMID 15505171. S2CID 24418905.
- Rogaeva E, Johnson J, Lang AE, Gulick C, Gwinn-Hardy K, Kawarai T, Sato C, Morgan A, Werner J, Nussbaum R, Petit A, Okun MS, McInerney A, Mandel R, Groen JL, Fernandez HH, Postuma R, Foote KD, Salehi-Rad S, Liang Y, Reimsnider S, Tandon A, Hardy J, St George-Hyslop P, Singleton AB (2005). "Analysis of the PINK1 gene in a large cohort of cases with Parkinson disease". Archives of Neurology. 61 (12): 1898–904. doi:10.1001/archneur.61.12.1898. PMID 15596610.
- Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR (2005). "Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability". Proc. Natl. Acad. Sci. U.S.A. 102 (16): 5703–8. Bibcode:2005PNAS..102.5703B. doi:10.1073/pnas.0500617102. PMC 556294. PMID 15824318.
- Deng H, Le WD, Zhang X, Pan TH, Jankovic J (2005). "G309D and W437OPA PINK1 mutations in Caucasian Parkinson's disease patients". Acta Neurol. Scand. 111 (6): 351–2. doi:10.1111/j.1600-0404.2005.00383.x. PMID 15876334. S2CID 10669009.
- Li Y, Tomiyama H, Sato K, Hatano Y, Yoshino H, Atsumi M, Kitaguchi M, Sasaki S, Kawaguchi S, Miyajima H, Toda T, Mizuno Y, Hattori N (2005). "Clinicogenetic study of PINK1 mutations in autosomal recessive early-onset parkinsonism". Neurology. 64 (11): 1955–7. doi:10.1212/01.WNL.0000164009.36740.4E. PMID 15955953. S2CID 46024206.