PNKD
PNKD is the abbreviation for a human neurological movement disorder paroxysmal nonkinesiogenic dyskinesia. Like many other human genetics disorders, PNKD also refers to the disease, the disease gene and the encoded protein. (PNKD) is a protein that in humans is encoded by the PNKD gene.[5][6] Alternative splicing results in the transcription of three isoforms. The mouse ortholog is called brain protein 17 (Brp17).
| PNKD | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PNKD, BRP17, DYT8, FPD1, KIPP1184, MR-1, MR1, PDC, PKND1, TAHCCP2, FKSG19, paroxysmal nonkinesigenic dyskinesia, PNKD1, MBL domain containing, MR-1S, PNKD metallo-beta-lactamase domain containing, R1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 609023 MGI: 1930773 HomoloGene: 75045 GeneCards: PNKD | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Structure
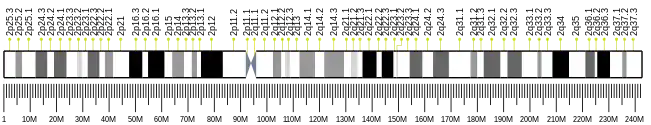
This gene is located on chromosome 2 at the band 2q35 and contains 12 exons.[6] At least three isoforms of varying lengths (long, medium, and short) can be produced by alternative splicing of this gene. While the gene products of the long (PNKD-L) and medium (PNKD-M) isoforms contain the C-terminal β-lactamase domain, the short (PNKD-S) isoform, commonly referred to as myofibrillogenesis regulator-1 (MR-1), contains only three exons and lacks homology to any known protein.[7][8] These isoforms also differ in their tissue-specific expression and subcellular localization. Specifically, PNKD-L is only expressed in the central nervous system whereas PNKD-M and PNKD-S are ubiquitously expressed across tissues.[7] Moreover, PNKD-L localizes to the cell membrane, PNKD-S to the cytoplasm and nucleus, and PNKD-M to the mitochondrion.[9]
Function
The function of PNKD proteins are unknown but the long and medium isoforms of PNKD contain a conserved β-lactamase domain which suggest it may function as an enzyme. The closest mammalian homolog to PNKD is HAGH, an enzyme involves in a two-step reaction to hydrolyze SLG and produce D-lactic acid and reduced GSH. However, the hydrolytic activity of PNKD is minimal.[7]
The long form of PNKD is neuronal specific and encodes a synaptic protein that localizes dominantly to the pre-synaptic membrane. Post-synaptic area and vesicular structure also occasionally has PNKD long form. PNKD long form interacts with pre-synaptic protein RIM and inhibits synaptic exocytosis. PNKD with disease mutations is less effective in inhibition thus the synaptic release is increased. This would cause excessive neurotransmitter release in the brain and maybe the root cause for triggering epilepsy in PNKD patients.[10]
Clinical significance
Point mutations in PNKD exon 1 cause an inherited neurological movement disorder in humans called paroxysmal nonkinesigenic dyskinesia.[6] Overexpression of PNKD has also been associated with multiple cancers, including pancreatic ductal adenocarcinoma,[11] gastric cancer,[12] ovarian cancer,[13] and breast cancer[8] and may serve as a therapeutic target for treating these cancers or a biomarker for assessing patient outcomes. The signaling pathways involved may vary depending on the cancer. For instance, in human breast cancer (MCF7) cells, PNKD may promote tumor cell proliferation by activating the MEK/ERK signaling pathway, while in human hepatoma (HepG2) cells, PNKD may operate through the MLC2/FAK/AKT pathway.[8]
References
- GRCh38: Ensembl release 89: ENSG00000127838 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000026179 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Fink JK, Rainer S, Wilkowski J, Jones SM, Kume A, Hedera P, Albin R, Mathay J, Girbach L, Varvil T, Otterud B, Leppert M (July 1996). "Paroxysmal dystonic choreoathetosis: tight linkage to chromosome 2q". American Journal of Human Genetics. 59 (1): 140–5. PMC 1915128. PMID 8659518.
- "Entrez Gene: PNKD paroxysmal nonkinesiogenic dyskinesia".
- Shen Y, Lee HY, Rawson J, Ojha S, Babbitt P, Fu YH, Ptácek LJ (June 2011). "Mutations in PNKD causing paroxysmal dyskinesia alters protein cleavage and stability". Human Molecular Genetics. 20 (12): 2322–32. doi:10.1093/hmg/ddr125. PMC 3098736. PMID 21487022.
- Gong Y, He H, Liu H, Zhang C, Zhao W, Shao RG (August 2014). "Phosphorylation of myofibrillogenesis regulator-1 activates the MAPK signaling pathway and induces proliferation and migration in human breast cancer MCF7 cells". FEBS Letters. 588 (17): 2903–10. doi:10.1016/j.febslet.2014.07.018. PMID 25066297. S2CID 38906043.
- "PNKD - Probable hydrolase PNKD - Homo sapiens (Human) - PNKD gene & protein". www.uniprot.org. Retrieved 2016-07-25.
- Shen Y, Ge WP, Li Y, Hirano A, Lee HY, Rohlmann A, Missler M, Tsien RW, Jan LY, Fu YH, Ptáček LJ (March 2015). "Protein mutated in paroxysmal dyskinesia interacts with the active zone protein RIM and suppresses synaptic vesicle exocytosis". Proceedings of the National Academy of Sciences of the United States of America. 112 (10): 2935–41. Bibcode:2015PNAS..112.2935S. doi:10.1073/pnas.1501364112. PMC 4364199. PMID 25730884.
- Zhao CY, Guo ZJ, Dai SM, Zhang Y, Zhou JJ (October 2013). "Clinicopathological and prognostic significance of myofibrillogenesis regulator-1 protein expression in pancreatic ductal adenocarcinoma". Tumour Biology. 34 (5): 2983–7. doi:10.1007/s13277-013-0862-4. PMID 23696030. S2CID 6019528.
- Guo J, Dong B, Ji JF, Wu AW (October 2012). "Myofibrillogenesis regulator-1 overexpression is associated with poor prognosis of gastric cancer patients". World Journal of Gastroenterology. 18 (38): 5434–41. doi:10.3748/wjg.v18.i38.5434. PMC 3471113. PMID 23082061.
- Lu RQ, Sun M, Gao X, Guo L (March 2012). "[Expression of a novel biomarker, MR-1S, in ovarian carcinoma and its biological significance]". Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology]. 34 (3): 176–81. doi:10.3760/cma.j.issn.0253-3766.2012.03.004. PMID 22780969.