PSMA scan
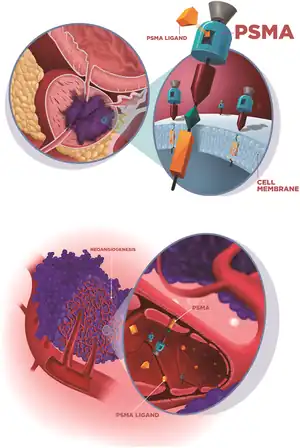
A PSMA scan is a nuclear medicine imaging technique used in the diagnosis and staging of prostate cancer. It is carried out by injection of a radiopharmaceutical with a positron or gamma emitting radionuclide and a prostate-specific membrane antigen (PSMA) targeting ligand. After injection, imaging of positron emitters such as gallium-68 (68Ga), copper-64 (64Cu), and fluorine-18 (18F) is carried out with a positron emission tomography (PET) scanner. For gamma emitters such as technetium-99m (99mTc) and indium-111 (111In) single-photon emission computed tomography (SPECT) imaging is performed with a gamma camera.
As well as the diagnosis and staging of prostate cancer, PSMA imaging can also be used to assess suitability for and plan treatment with external beam radiotherapy and PSMA-targeted radionuclide therapy.[1][2]
Mechanism
Attempts have been made to target the overexpression of PSMA in prostate cancer cells for several decades, although PSMA is also found in other tissue. PSMA targeting molecules have included antibodies, aptamers, peptides, and small-molecule inhibitors.[3][4] Initially, development focussed on the antibody capromab. Later research has focussed on small molecule ligands that bind to the extracellular active centre of PSMA, such as PSMA-11.[5] These ligands for PSMA-scanning target the large extracellular region of the PSMA glycoprotein.[6]
PSMA however is also over-expressed in non prostate cancer cells, including kidney, salivary gland, lacrimal gland and duodenal mucosa, where physiological uptake may be seen on imaging.[7]
Clinical use
European Association of Urology (EAU) guidelines recognise that PSMA can provide accurate staging, however there is a lack of outcome data to inform further management.[8] The American Society of Clinical Oncology (ASCO) guidelines for imaging of advanced prostate cancer also recommend PSMA imaging (among other PET radiopharmaceuticals), while acknowledging that these are not FDA approved and therefore limited to a clinical trial or other controlled research setting.[9]
Radiopharmaceuticals
| Radionuclide | Name | INN | Brand name(s) | FDA approval | EMA approval | References |
|---|---|---|---|---|---|---|
| 18F | DCFPyL | PIFLUFOLASTAT F-18 | Pylarify | Yes | [10] | |
| 18F | PSMA-1007 | |||||
| 68Ga | PSMA-11 | Gallium (68Ga) gozetotide |
| Yes | Yes | [3] |
| 68Ga | THP-PSMA | Gallium (68Ga) gozetotide | GalliProst | [11] | ||
| 99mTc | PSMA-11 | |||||
| 99mTc | iPSMA | TLX599-CDx | [12] | |||
| 111In | capromab pendetide | Prostascint | Yes | [13] | ||
| 111In | PSMA-I&T | [3] | ||||
Availability
In part thanks to the wide range of similar PSMA radiopharmaceuticals,[4][14] approval by regulatory authorities is at varying stages. Even so, use has been widespread in some areas, particularly as part of clinical trials. For example, European Association of Urology (EAU) guidelines have included recommendations to perform PSMA PET scans in certain circumstances since 2018, and there has been widespread agreement of the utility of PSMA scanning for several years.[15][16][17][18]
Australia
A kit for manufacture of a 68Ga-PSMA-11 product, branded Illucix, was approved by Australia's Therapeutic Goods Administration (TGA) in 2021.[19]
Europe
A marketing authorisation application for 68Ga-PSMA-11 (INN Gallium (68Ga) gozetotide), under the brand name Illucix, was made to the Danish Medicines Agency, on behalf of several EU countries and the UK. Approval is expected in 2022.[20][21]
In 2022 a marketing authorisation application was made by the manufacturer of 18F-DCFPyL (branded Pylarify) to the European Medicines Agency.[22]
Polish manufacturer and distributor of radiopharmaceutical procuts, Polatom, has been granted a US patent for a 99mTc-PSMA-T4 kit.[23][24] In the UK, Tc-99m labelled PSMA has product authorisation but lacks funding.[25]
Canada
A new drug submission was made to Canada's regulator in 2021, for 68Ga-PSMA-11.[26]
United States
The first approved PSMA imaging agent was indium-111 (111In) capromab pendetide (branded Prostascint). It received Food and Drug Administration (FDA) approval in 1996.[13] However, the agent had poor sensitivity and saw little widespread use.[17]
The first PET PSMA imaging agent, 68Ga-PSMA-11, was approved by the FDA in 2020.[27] Listed indications include suspected metastasis prior to initial treatment, and recurrence of prostate cancer (based on elevated serum prostate-specific antigen (PSA) level).[28] This was followed by two further 68Ga-PSMA-11 agents in 2021 and 2022 (branded Illucix and Locametz).[29][30] Listed indications for Lucametz additionally includes selection of patients prior to 177Lu-PSMA radionuclide therapy.[31]
An 18F-PSMA agent (18F-DCFPyL) was approved by the FDA in 2021.[32] Indications are as for 68Ga-PSMA-11.[33][34]
References
- Wang, Fujin; Li, Zhifeng; Feng, Xiaoqian; Yang, Dazhuang; Lin, Mei (March 2022). "Advances in PSMA-targeted therapy for prostate cancer". Prostate Cancer and Prostatic Diseases. 25 (1): 11–26. doi:10.1038/s41391-021-00394-5. PMID 34050265. S2CID 235241529.
- Mena, Esther; Lindenberg, Liza; Choyke, Peter (March 2022). "The Impact of PSMA PET/CT Imaging in Prostate Cancer Radiation Treatment". Seminars in Nuclear Medicine. 52 (2): 255–262. doi:10.1053/j.semnuclmed.2021.12.008. PMC 8960055. PMID 35016755.
- Wang, He; He, Zhangxin; Liu, Xiao-Ang; Huang, Yuhua; Hou, Jianquan; Zhang, Weijie; Ding, Dan (August 2022). "Advances in Prostate‐Specific Membrane Antigen (PSMA)‐Targeted Phototheranostics of Prostate Cancer". Small Structures. 3 (8): 2200036. doi:10.1002/sstr.202200036. S2CID 248702668.
- Piron, Sarah; Verhoeven, Jeroen; Vanhove, Christian; De Vos, Filip (March 2022). "Recent advancements in 18F-labeled PSMA targeting PET radiopharmaceuticals". Nuclear Medicine and Biology. 106–107: 29–51. doi:10.1016/j.nucmedbio.2021.12.005. PMID 34998217.
- Hofman, Michael S.; Hicks, Rodney J.; Maurer, Tobias; Eiber, Matthias (January 2018). "Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls". RadioGraphics. 38 (1): 200–217. doi:10.1148/rg.2018170108. PMID 29320333.
- de Galiza Barbosa, Felipe; Queiroz, Marcelo Araujo; Nunes, Rafael Fernandes; Costa, Larissa Bastos; Zaniboni, Elaine Caroline; Marin, José Flavio Gomes; Cerri, Giovanni Guido; Buchpiguel, Carlos Alberto (December 2020). "Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings". Cancer Imaging. 20 (1): 23. doi:10.1186/s40644-020-00300-7. PMC 7071711. PMID 32169115.
- Heidegger, Isabel; Kesch, Claudia; Kretschmer, Alexander; Tsaur, Igor; Ceci, Francesco; Valerio, Massimo; Tilki, Derya; Marra, Giancarlo; Preisser, Felix; Fankhauser, Christian D; Zattoni, Fabio; Chiu, Peter; Puche-Sanz, Ignacio; Olivier, Jonathan; van den Bergh, Roderik C N; Kasivisvanathan, Veeru; Pircher, Andreas; Virgolini, Irene; Gandaglia, Giorgio (January 2022). "Biomarkers to personalize treatment with 177Lu-PSMA-617 in men with metastatic castration-resistant prostate cancer - a state of the art review". Therapeutic Advances in Medical Oncology. 14: 175883592210819. doi:10.1177/17588359221081922. PMC 8902011. PMID 35273651.
- "EAU Guidelines on Prostate Cancer". Uroweb. European Association of Urology. 2022. Retrieved 8 December 2022.
- Trabulsi, Edouard J.; Rumble, R. Bryan; Jadvar, Hossein; Hope, Thomas; Pomper, Martin; Turkbey, Baris; Rosenkrantz, Andrew B.; Verma, Sadhna; Margolis, Daniel J.; Froemming, Adam; Oto, Aytekin; Purysko, Andrei; Milowsky, Matthew I.; Schlemmer, Heinz-Peter; Eiber, Matthias; Morris, Michael J.; Choyke, Peter L.; Padhani, Anwar; Oldan, Jorge; Fanti, Stefano; Jain, Suneil; Pinto, Peter A.; Keegan, Kirk A.; Porter, Christopher R.; Coleman, Jonathan A.; Bauman, Glenn S.; Jani, Ashesh B.; Kamradt, Jeffrey M.; Sholes, Westley; Vargas, H. Alberto (10 June 2020). "Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline". Journal of Clinical Oncology. 38 (17): 1963–1996. doi:10.1200/JCO.19.02757. PMID 32048940.
- "PSMA Radiopharmaceutical Effective against Prostate Cancer". National Cancer Institute. National Institutes of Health. 29 June 2021.
- Neels, Oliver C; Kopka, Klaus; Liolios, Christos; Afshar-Oromieh, Ali (13 December 2021). "Radiolabeled PSMA Inhibitors". Cancers. 13 (24): 6255. doi:10.3390/cancers13246255. PMC 8699044. PMID 34944875.
- Ferro-Flores, Guillermina; Luna-Gutiérrez, Myrna; Ocampo-García, Blanca; Santos-Cuevas, Clara; Azorín-Vega, Erika; Jiménez-Mancilla, Nallely; Orocio-Rodríguez, Emmanuel; Davanzo, Jenny; García-Pérez, Francisco O (May 2017). "Clinical translation of a PSMA inhibitor for 99m Tc-based SPECT". Nuclear Medicine and Biology. 48: 36–44. doi:10.1016/j.nucmedbio.2017.01.012. PMID 28193503.
- "PROSTASCINT". Drugs@FDA. Food and Drug Administration. Retrieved 19 January 2023.
- Young, Jennifer D; Jauregui-Osoro, Maite; Wong, Wai-Lup; Cooper, Margaret S; Cook, Gary; Barrington, Sally F; Ma, Michelle T; Blower, Philip J; Aboagye, Eric O (December 2021). "An overview of nuclear medicine research in the UK and the landscape for clinical adoption". Nuclear Medicine Communications. 42 (12): 1301–1312. doi:10.1097/MNM.0000000000001461. PMC 8584216. PMID 34284442.
- EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer (PDF) (Report). European Association of Urology. 2018. Retrieved 23 July 2022.
- "EAU Guidelines - Prostate Cancer - Summary of Changes 2018". Uroweb. European Association of Urology. Retrieved 23 July 2022.
- Tagawa, Scott T; Bander, Neil H; Osborne, Joseph R (2 March 2022). "Evaluating the Current Role of PSMA PET: Utility, Availability, and Challenges". ASCO Daily News. American Society of Clinical Oncology. doi:10.1200/ADN.22.200864.
- Fanti, Stefano; Goffin, Karolien; Hadaschik, Boris A; Herrmann, Ken; Maurer, Tobias; MacLennan, Steven; Oprea-Lager, Daniela E.; Oyen, Wim JG; Rouvière, Olivier; Mottet, Nicolas; Bjartell, Anders (February 2021). "Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer". European Journal of Nuclear Medicine and Molecular Imaging. 48 (2): 469–476. doi:10.1007/s00259-020-04934-4. PMC 7835167. PMID 32617640.
- "Illuccix". Therapeutic Goods Administration. Australian Government Department of Health. 24 November 2021. Retrieved 23 July 2022.
- "Submission of European Marketing Authorisation Application". Telix. 1 May 2020. Retrieved 23 July 2022.
- "Telix's (ASX:TLX) Illuccix EU regulatory submission progresses to final stage". The Market Herald. 9 December 2021.
- "Curium Announces The Submission Of Its Marketing Authorization Application For [18F]-DCFPyL To The European Medicines Agency" (Press release). Curium. 27 June 2022.
- "POLATOM with a patent". Polatom. 3 October 2022.
- US patent 11426395B2, Arkadiusz Eugeniusz Sikora,Michal Maurin,Antoni Wlodzimierz Jaron,Justyna Pijarowska-Kruszyna,Monika Wyczólkowska,Barbara Janota,Marcin Radzik,Piotr Garnuszek,Urszula Karczmarczyk, "PSMA inhibitor derivatives for labelling with 99mTc via HYNIC, a radiopharmaceutical kit, radiopharmaceutical preparations and their use in prostate cancer diagnostics", published 2022-08-30, issued 2022-08-30, assigned to NARODOWE CENTRUM BADAN JADROWYCH OSRODEK RADIOIZOTOPOW POLATOM
- "Review of molecular radiotherapy services in the UK". The Royal College of Radiologists. RCP, IPEM, BNMS, RCR. 2021. p. 25.
- "Drug and Health Product Submissions Under Review (SUR): New drug submissions under review". Health Canada. 10 March 2021. Retrieved 23 July 2022.
- "Drug Approval Package: Gallium Ga 68 PSMA-11". U.S. Food and Drug Administration (FDA). 16 December 2020. Archived from the original on 26 January 2021. Retrieved 25 December 2020.
- "Gallium Ga 68 PSMA-11 label" (PDF). Drugs@FDA. Food and Drug Administration. December 2020. Retrieved 23 July 2022.
- "ILLUCCIX". Drugs@FDA. Food and Drug Administration. Retrieved 23 July 2022.
- "Drug Approval Package: LOCAMETZ". Drugs@FDA. Food and Drug Administration. Retrieved 23 July 2022.
- "Locametz label" (PDF). Drugs@FDA. Food and Drug Administration. March 2022. Retrieved 23 July 2022.
- "Drug Approval Package: PYLARIFY". Drugs@FDA. Food and Drug Administration. Retrieved 23 July 2022.
- "Pylarify label" (PDF). Drugs@FDA. Food and Drug Administration. May 2021. Retrieved 23 July 2022.
- Jadvar, Hossein (24 May 2022). "Prostate-specific Membrane Antigen PET: Standard Imaging in Prostate Cancer". Radiology. 304 (3): 609–610. doi:10.1148/radiol.221074. PMC 9434807. PMID 35608452.
- "Grupo RPH fecha parceria para fabricação de radiofármaco no Brasil". Medicina S/A (in Brazilian Portuguese). 16 December 2021.
- "Illuccix Granted Use Authorisation for Prostate Cancer Imaging in Brazil". BioSpace (Press release). Telix Pharmaceuticals. 30 November 2021. Retrieved 23 July 2022.