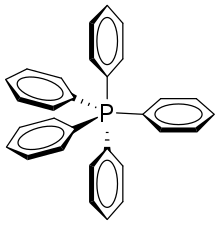
Pentaphenylphosphorus
Pentaphenylphosphorus is an organic phosphorane containing five phenyl groups connected to a central phosphorus atom. The phosphorus atom is considered to be in the +5 oxidation state. The chemical formula could be written as P(C6H5)5 or Ph5P, where Ph represents the phenyl group. It was discovered and reported in 1949 by Georg Wittig.[2]
 | |
| Names | |
|---|---|
| IUPAC name
pentakis-phenyl-λ5-phosphane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H25P | |
| Molar mass | 416.504 g·mol−1 |
| Appearance | colourless[1] |
| Density | 1.22 |
| Related compounds | |
Other cations |
pentaphenylarsenic pentaphenylantimony pentaphenylbismuth |
Related compounds |
triphenylphosphine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Formation
Pentaphenylphosphorus can be formed by the action of phenyllithium on tetraphenylphosphonium bromide[3] or tetraphenylphosphonium iodide.[2]
Properties
In the solid form of pure pentaphenylphosphorus, molecules have a trigonal bipyramid shape. The crystal structure of the pure form is monoclinic with a=10.03, b=17.22 c=14.17 Å and β=112.0°. The unit cell volume is 2267.5 Å3. There are four of the formula per unit cell (Z=4). Space group is Cc.[4] Pentaphenyl phosphorus can also crystallise with some of the solvent, (to form a solvate). With tetrahydrofuran, the crystal structure of which is triclinic, with space group P1, with unit cell dimensions a=10.095 b=10.252 c=12.725 Å α=71.21, β=76.98, γ=87.12 with Z=2.[5] It can also form a solvate crystal with cyclohexane[6] or benzene.[7]
Reactions
On heating, pentaphenylphosphorus decomposes to form biphenyl and triphenylphosphine.[2]
Pentaphenylphosphorus reacts with acidic hydrogen to yield the tetraphenylphosphonium ion and benzene.[2] For example pentaphenylphosphorus reacts with carboxylic acids and sulfonic acids to yield the tetraphenylphosphonium salt of the carboxylate or sulfonate, and benzene.[8]
Pentaphenylphosphorus transfers a phenyl group to organomercury, and tin halides. For example pentaphenylphosphorus reacts with phenylmercury chloride to yield diphenyl mercury and tetraphenylphosphonium chloride. With tributyltin chloride, tributylphenyltin is produced. However the pentaphenylphosphorus reaction with triphenylbismuth difluoride, chloride or bromide makes triphenylbismuth and fluorobenzene, chlorobenzene or bromobenzene. This is probably because tetraphenylbismuth halides (Ph4BiF, Ph4BiCl, Ph4BiBr) spontaneously decompose as the halogen reacts with one phenyl group.[9]
When heated with carbon dioxide or sulfur, bicyclic compounds are formed, where the reactant bridges between one of the phenyl groups and the phosphorus.[10]
References
- Freeman, B.H.; Lloyd, D.; Singer, M.I.C. (January 1972). "Tetraphenylcyclopentadienylides". Tetrahedron. 28 (2): 343–352. doi:10.1016/0040-4020(72)80141-8.
- Wittig, Georg; Rieber, Martin (1949-05-10). "Über die Metallierbarkeit von quaternären Ammonium- und Phosphonium-Salzen". Justus Liebigs Annalen der Chemie. 562 (3): 177–186. doi:10.1002/jlac.19495620303.
- Seyferth, Dietmar.; Fogel, Joseph S.; Heeren, James K. (January 1964). "The Reaction of Vinyllithium with Tetraphenylphosphonium Bromide and the Formation of Phosphinemethylenes by RLi Addition to Vinylphosphonium Halides". Journal of the American Chemical Society. 86 (2): 307–308. doi:10.1021/ja01056a059.
- Wheatley, P. J. (1964). "408. The crystal and molecular structure of pentaphenylphosphorus". Journal of the Chemical Society (Resumed): 2206. doi:10.1039/JR9640002206.
- Müller, Gerhard; Bildmann, Ulrich Jürgen (2004-12-01). "Crystal and Molecular Structure of P(C 6 H 5 ) 5 · 0.5 THF". Zeitschrift für Naturforschung B. 59 (11–12): 1411–1414. doi:10.1515/znb-2004-11-1207. S2CID 99733089.
- Brock, C. P. (1977-11-01). "Lattice energy calculations for (C 6 H 5 ) 5 M 0.5C 6 H 12 , M = P, As and Sb: towards an understanding of crystal packing in the pentaphenyl group V compounds". Acta Crystallographica Section A. 33 (6): 898–902. Bibcode:1977AcCrA..33..898B. doi:10.1107/S0567739477002204.
- Губанова, Юлия Олеговна; Шарутина, Ольга Константиновна (2020-08-16). "Синтез и строение карбоксилатов тетрафенилфосфония [Ph 4 P][OC(O)C 6 H 3 (OH) 2 -2,6], [Ph 4 P][OC(O)CH 2 CH 2 C(O)OH]" [Synthesis and Structure of Tetraphenylphosphonium Carboxylates [Ph4P][OC(O)C6H3 (OH) 2-2,6], [Ph4P][OC(O)CH2CH2C(O)OH]]. Химия (in Russian). 12 (3): 79–87.
- Shaturin, V. V.; Senchurin, V. S.; Shaturina, O. K.; Boyarkina, E. A. (January 2009). "Tetraphenylphosphonium carboxylates and sulfonates. Synthesis and structure". Russian Journal of General Chemistry. 79 (1): 78–87. doi:10.1134/S1070363209010125. S2CID 96900890.
- Sharutin, V. V.; Sharutina, O. K.; Senchurin, V. S.; Egorova, I. V.; Ivanenko, T. K.; Petrov, B. I. (2003). "Phenylation of Organic Derivatives of Mercury, Silicon, Tin, and Bismuth with Pentaphenylantimony and Pentaphenylphosphorus". Russian Journal of General Chemistry. 73 (2): 202–203. doi:10.1023/A:1024731719528. S2CID 91420871.
- C. D. Hall (1990). "Pentaco-ordinated and Hexaco-ordinated Compounds". In B. J. Walker (ed.). Organophosphorus Chemistry. Royal Society of Chemistry. pp. 51–54. ISBN 978-0-85186-196-8.
Extra reading
- Zykova, A.R. (2021). "РЕАКЦИЯ ПЕНТАФЕНИЛФОСФОРА С ГЕКСАХЛОРОПЛАТИНОВОЙ КИСЛОТОЙ" [Reaction of Pentaphenylphosphorus with Hexachloroplatinic Acid]. Bulletin of the South Ural State University Series "Chemistry" (in Russian). 13 (1): 31–38. doi:10.14529/chem210103.
- Hoffmann, Roald; Howell, James M.; Muetterties, Earl L. (May 1972). "Molecular orbital theory of pentacoordinate phosphorus" (PDF). Journal of the American Chemical Society. 94 (9): 3047–3058. doi:10.1021/ja00764a028.