Peroxomonosulfate
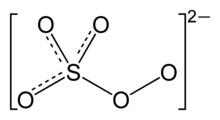
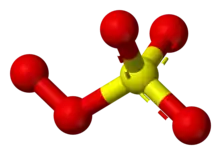
The peroxomonosulfate ion, SO2−
5, is a sulfur oxoanion. It is sometimes referred to as the persulfate ion, but this term also refers to the peroxydisulfate ion, S
2O2−
8.
 | |
 | |
| Names | |
|---|---|
| Other names
Persulfate[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 101038 | |
| |
| |
| Properties | |
| O5S−2 | |
| Molar mass | 112.06 g·mol−1 |
| Conjugate acid | Peroxymonosulfuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Its other IUPAC names are sulfuroperoxoate and trioxidoperoxidosulfate(2−).[2]
Compounds containing peroxomonosulfate
- Na2SO5
- KHSO5
See also
References
- Ambiguous—see persulfate
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. pp. 139,328. Electronic version.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.