Peterson olefination
| Peterson olefination | |
|---|---|
| Named after | Donald John Peterson |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | peterson-olefination |
| RSC ontology ID | RXNO:0000080 |
The Peterson olefination (also called the Peterson reaction) is the chemical reaction of α-silyl carbanions (1 in diagram below) with ketones (or aldehydes) to form a β-hydroxysilane (2) which eliminates to form alkenes (3).[1]
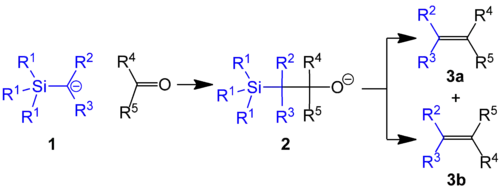
Reaction mechanism
One attractive feature of the Peterson olefination is that it can be used to prepare either cis- or trans-alkenes from the same β-hydroxysilane. Treatment of the β-hydroxysilane with acid will yield one alkene, while treatment of the same β-hydroxysilane with base will yield the alkene of opposite stereochemistry.
Basic elimination
The action of base upon a β-hydroxysilane (1) results in a concerted syn elimination of (2) or (3) to form the desired alkene. The penta-coordinate silicate intermediate (3) is postulated, but no proof exists to date.
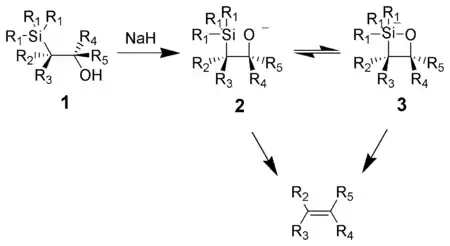
Potassium alkoxides eliminate quickly, while sodium alkoxides generally require heating. Magnesium alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen, hence increasing the alkoxide nucleophilicity.
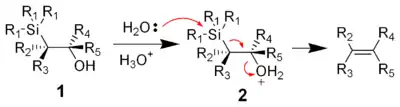
Acidic elimination
The treatment of the β-hydroxysilane (1) with acid results in protonation and an anti elimination to form the desired alkene.
Alkyl substituents
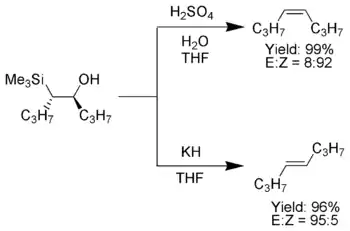
When the α-silyl carbanion contains only alkyl, hydrogen, or electron-donating substituents, the stereochemical outcome of the Peterson olefination can be controlled,[7] because at low temperature the elimination is slow and the intermediate β-hydroxysilane can be isolated.
Once isolated, the diastereomeric β-hydroxysilanes are separated. One diastereomer is treated with acid, while the other is treated with base, thus converted the material to an alkene with the required stereochemistry.[4]
Electron-withdrawing substituents
When the α-silyl carbanion contains electron-withdrawing substituents, the Peterson olefination directly forms the alkene. The intermediate β-hydroxysilane cannot be isolated as it eliminates in-situ. The basic elimination pathway has been postulated in these cases.
Variations
Acidic elimination conditions are sometimes not feasible as the acid also promotes double bond isomerization. Additionally, elimination using sodium or potassium hydride may not be feasible due to incompatible functional groups. Chan et al. have found that acylation of the intermediate silylcarbinol with either acetyl chloride or thionyl chloride gives a β-silyl ester that will eliminate spontaneously at 25 °C giving the desired alkene.[8] Corey and co-workers developed a method (sometimes dubbed the Corey-Peterson olefination[9]) using a silylated imine to yield an α,β-unsaturated aldehyde from a carbonyl compound in one step.[10] For an example for its use in total synthesis see: Kuwajima Taxol total synthesis
References
- D. J. Peterson (1968). "Carbonyl olefination reaction using silyl-substituted organometallic compounds". J. Org. Chem. 33 (2): 780–784. doi:10.1021/jo01266a061.
- Birkofer, L.; Stiehl, O. Top. Curr. Chem. 1980, 88, 58. (Review)
- Ager, D. J. Synthesis 1984, 384–398. (Review)
- Ager, D. J. Org. React. 1990, 38, 1. doi:10.1002/0471264180.or038.01
- T. H. Chan (1977). "Alkene synthesis via β-functionalized organosilicon compounds". Acc. Chem. Res. 10 (12): 442–448. doi:10.1021/ar50120a003.
- New developments in the Peterson olefination reaction L. Frances van Staden, David Gravestock and David J. Ager Chem. Soc. Rev., 2002,31, 195-200 doi:10.1039/A908402I
- Barrett, A. G. M.; Flygare, J. A.; Hill, J. M.; Wallace, E. M. (1998). "Stereoselective Alkene Synthesis via 1-Chloro-1-[(dimethyl)phenylsilyl]alkanes and α-(Dimethyl)phenylsilyl Ketones: 6-Methyl-6-dodecene". Organic Syntheses.; Collective Volume, vol. 9, p. 580
- T. H. Chan & E. Chang (1974). "Synthesis of alkenes from carbonyl compounds and carbanions alpha to silicon. III. Full report and a synthesis of the sex pheromone of gypsy moth". J. Org. Chem. 39 (22): 3264–3268. doi:10.1021/jo00936a020. PMID 4473100.
- X. Zeng; F. Zeng & E. Negishi (2004). "Efficient and Selective Synthesis of 6,7-Dehydrostipiamide via Zr-Catalyzed Asymmetric Carboalumination and Pd-Catalyzed Cross-Coupling of Organozincs". Org. Lett. 6 (19): 3245–3248. doi:10.1021/ol048905v. PMID 15355023.
- E. J. Corey; D. Enders & M. G. Bock (1976). "A simple and highly effective route to α-β-unsaturated aldehydes". Tetrahedron Letters. 17 (1): 7–10. doi:10.1016/S0040-4039(00)71308-6.