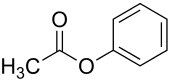
Phenyl acetate
Phenyl acetate is the ester of phenol and acetic acid. It can be produced by reacting phenol (Which can be produced by decarboxylation of aspirin[2]) with acetic anhydride or acetyl chloride.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Phenyl acetate | |
| Systematic IUPAC name
Phenyl ethanoate | |
| Other names
Phenol acetate (Acetyloxy)benzene Acetoxybenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.160 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
| Density | 1.075 g/mL[1] |
| Melting point | −30 °C (−22 °F; 243 K) |
| Boiling point | 195–196 °C (383–385 °F; 468–469 K)[1] |
| -82.04·10−6 cm3/mol | |
| Hazards | |
| Flash point | 76 °C (169 °F; 349 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phenyl acetate can be separated into phenol and an acetate salt, via saponification: heating the phenyl acetate with a strong base, such as sodium hydroxide, will produce phenol and an acetate salt (sodium acetate, if sodium hydroxide were used).
References
- Phenyl acetate, Alfa Aesar
- Seo, Sangwon; Taylor, John B.; Greaney, Michael F. (2012-07-23). "Protodecarboxylation of benzoic acids under radical conditions". Chemical Communications. 48 (66): 8270–8272. doi:10.1039/C2CC33306F. ISSN 1364-548X.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.