Phosphorine
Phosphorine (IUPAC name: phosphinine) is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Phosphinine[1] | |||
| Other names
Phosphabenzene | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
| MeSH | Phosphinine | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H5P | |||
| Molar mass | 96.069 g·mol−1 | ||
| Related compounds | |||
Related -ines |
|||
Related compounds |
Phosphole | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Phosphorine is an air-sensitive oil[2] but is otherwise stable when handled using air-free techniques (however, substituted derivatives can often be handled under air without risk of decomposition).[3][4] In contrast, silabenzene, a related heavy-element analogue of benzene, is not only air- and moisture-sensitive but also thermally unstable without extensive steric protection.
History
The first phosphorine to be isolated is 2,4,6-triphenylphosphorine. It was synthesized by Gottfried Märkl in 1966 by condensation of the corresponding pyrylium salt and phosphine or its equivalent ( P(CH2OH)3 and P(SiMe3)3).[3]
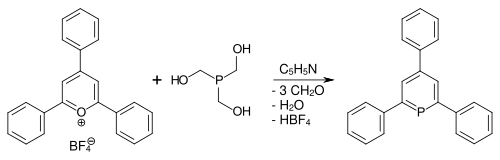
The (unsubstituted) parent phosphorine was reported by Arthur J. Ashe III in 1971.[2][5] Ring-opening approaches have been developed from phospholes.[6]
Structure, bonding, and properties
Structural studies by electron diffraction reveal that phosphorine is a planar aromatic compound with 88% of aromaticity of that of benzene. Potentially relevant to its high aromaticity are the well matched electronegativities of phosphorus (2.1) and carbon (2.5). The P–C bond length is 173 pm and the C–C bond lengths center around 140 pm and show little variation.[7]
 Bond lengths and angles of benzene, pyridine, phosphorine, arsabenzene, stibabenzene and bismabenzene |
Although phosphorine and pyridine are structurally similar, phosphorines are far less basic. The pKa of C5H5PH+ and C5H5NH+ are respectively −16.1 and +5.2.[6] Methyllithium adds to phosphorus in phosphorine whereas it adds to the 2-position of pyridine.[8]
Phosphorine undergoes electrophilic substitution reactions like ordinary aromatic compounds: bromination, acylation, and so on.
Coordination chemistry
Coordination complexes bearing phosphorine as a ligand are known. Phosphorines can bind to metals through phosphorus center. Complexes of the diphospha analogue of 2,2′-bipyridine are known. Phosphorines also form pi-complexes, illustrated by V(η6-C5H5P)2.[6]
See also
- Six-membered aromatic rings with one carbon replaced by an element from another group: borabenzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, stibabenzene, bismabenzene, pyrylium, thiopyrylium, selenopyrylium, telluropyrylium
References
- "CHAPTER P-1. General Principles, Rules, and Conventions". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 47. doi:10.1039/9781849733069-00001. ISBN 9780854041824. OCLC 1077224056.
- Ashe, A. J. (1971). "Phosphabenzene and Arsabenzene". Journal of the American Chemical Society. 93 (13): 3293–3295. doi:10.1021/ja00742a038.
- G. Märkl, 2,4,6-Triphenylphosphabenzol in Angewandte Chemie 78, 907–908 (1966)
- Newland, R. J.; Wyatt, M. F.; Wingad, R. L.; Mansell, S. M. (2017). "A ruthenium(II) bis(phosphinophosphinine) complex as a precatalyst for transfer-hydrogenation and hydrogen-borrowing reactions". Dalton Transactions. 46 (19): 6172–6176. doi:10.1039/C7DT01022B. ISSN 1477-9226. PMID 28436519.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 544. ISBN 978-0-08-037941-8.
- François Mathey "Phosphorus Heterocycles" in Modern Heterocyclic Chemistry, First Edition, edited by Julio Álvarez-Builla, Juan José Vaquero, José Barluenga, Wiley-VCH, Weinheim, 2011. doi:10.1002/9783527637737.ch23.
- László Nyulászi "Aromaticity of Phosphorus Heterocycles" Chem. Rev., 2001, volume 101, pp 1229–1246. doi:10.1021/cr990321x
- Ashe III, Arthur J.; Smith, Timothy W. "The reaction of phosphabenzene, arsabenzene and stibabenzene with methyllithium." Tetrahedron Letters 1977, volume 18, pp. 407–410. doi:10.1016/S0040-4039(01)92651-6
- Quin, L. D. (2000). A Guide to Organophosphorus Chemistry. Wiley-Interscience. ISBN 978-0-471-31824-8.