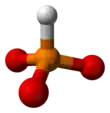
Phosphite anion
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphonate | |||
| Systematic IUPAC name
Phosphite[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| 68617 | |||
| MeSH | Phosphorite | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| HPO2− 3 | |||
| Molar mass | 79.9810 g mol−1 | ||
| Related compounds | |||
Other anions |
Phosphinite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Nomenclature
The IUPAC recommended name for phosphorous acid is phosphonic acid. Correspondingly, the IUPAC-recommended name for the HPO2−
3 ion is phosphonate. In the US the IUPAC naming conventions for inorganic compounds are taught at high school, but not as a 'required' part of the curriculum.[2] A well-known university-level textbook follows the IUPAC recommendations.[3] In practice any reference to "phosphite" should be investigated to determine the naming convention being employed.
Salts containing HPO32−, called phosphonates or phosphites
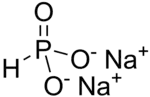 Structural formula of Na2HPO3. The anion has C3v symmetry.
Structural formula of Na2HPO3. The anion has C3v symmetry.
From the commercial perspective, the most important phosphite salt is basic lead phosphite. Many salts containing the phosphite ion have been investigated structurally, these include sodium phosphite pentahydrate (Na2HPO3·5H2O). (NH4)2HPO3·H2O, CuHPO3·H2O, SnHPO3 and Al2(HPO3)3·4H2O.[4] The structure of HPO2−
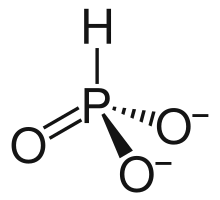
3 is approximately tetrahedral.[5][6]
HPO2−
3 has a number of canonical resonance forms making it isoelectronic with bisulfite ion, HSO−
3, which has a similar structure.[7]
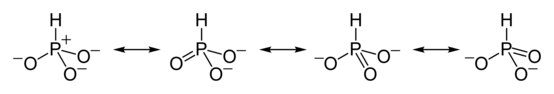
Salts containing HP(O)2OH−
Acid or hydrogen phosphites are called hydrogenphosphonates or acid phosphites. IUPAC recommends the name hydrogenphosphonates). They are anions HP(O)2OH−. Aypical derivative is the salt [NH4][HP(O)2OH].[7][6] Many related salts are known, e.g., RbHPHO3, CsHPHO3, TlHPHO3. These salts are prepared by treating phosphorous acid with the metal carbonate. These compounds contain a layer polymeric anion consisting of HPO3 tetrahedra linked by hydrogen bonds. These layers are interleaved by layers of metal cations.[8]
Organic esters of hydrogen phosphites are anions with the formula HP(O)2OR− (R = organic group). One commercial example is the fungicide fosetyl-Al with the formula [C2H5OP(H)O2]3Al.[9]
Salts containing H2P2O52−, called diphosphites or pyrophosphites
Pyrophosphites (diphosphites) can be produced by gently heating acid phosphites under reduced pressure. They contain the ion H
2P
2O2−
5, which can be formulated [HP(O)2O−P(O)2H]2−.[7][6]
Parallels in arsenic chemistry
In contrast to the paucity of evidence for PO3−
3, the corresponding arsenic ion, ortho-arsenite, AsO3−
3 is known. An example is Ag3AsO3 as well as the polymeric meta-arsenite (AsO−
2)
n.[7] The iso-electronic sulfite ion, SO2−
3 is known from its salts.[7]
Use as fungicides
Inorganic phosphites (containing HPO2−
3) have been applied to crops to combat fungus-like pathogens of the order oomycetes (water molds). The situation is confusing because of the similarity in name between phosphite and phosphate (a major plant nutrient and fertilizer ingredient), and controversial because phosphites have sometimes been advertised as fertilizers, even though they are converted to phosphate too slowly to serve as a plant's main phosphorus source. In fact, phosphites may cause phytotoxicity when a plant is starved of phosphates.[10] Lemoynie[11] and others have described this complicated situation and noted that calling phosphites fertilizers avoided the regulatory complication and negative public perceptions that might have been incurred by registering them as fungicides.[10]
A major form of inorganic phosphite used in agriculture is monopotassium phosphite. This compound does serve as a potassium fertilizer.
See also
- Hypophosphite – H
2PO−
2 - Organophosphorus
- Phosphine – PH3 and the organic phosphines PR3
- Phosphine oxide – OPR3
- Phosphinite – P(OR)R2
- Phosphonite – P(OR)2R
- Phosphinate – OP(OR)R2
- Phosphonate – organic phosphonates OP(OR)2R
- Phosphate – PO3−
4 - Organophosphate – OP(OR)3
Further reading
- A. Earnshaw; Norman Greenwood (1997). The Chemistry of the Elements (2nd ed.). pp. 513–514.
References
- "Phosphorite - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Physical setting/ chemistry core curriculum, The University of the State of New York, The State Education Department, http://www.p12.nysed.gov/ciai/mst/pub/chemist.pdf
- Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- "Synthesis and crystal structures of aluminum and iron phosphites", D.M. Poojary, Y. Zhang, D.E. Cox, P.R. Rudolf, S. Cheng & A. Clearfield, J. Chem. Crystallogr. 24 (1994) 155–163
- L. E. Gordon, W. T. A. Harrison. "Bis(melaminium) hydrogen phosphite tetrahydrate". Acta Crystallogr. 59 (2): o195–o197. doi:10.1107/S1600536803001247
- "Crystal chemistry of inorganic phosphites", J. Loub, Acta Crystallogr. (1991), B47, 468–473, doi:10.1107/S0108768191002380
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Kosterina EV, Troyanov SI, Kemnitz E, Aslanov LA (2001). "Synthesis and Crystal Structure of Acid Phosphites RbH2PO3, CsH2PO3, and TlH2PO3". Russian Journal of Coordination Chemistry. 27 (7): 458–462. doi:10.1023/A:1011377229855. S2CID 91297300.
- Franz Müller; Peter Ackermann; Paul Margot (2012). "Fungicides, Agricultural, 2. Individual Fungicides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o12_o06. ISBN 978-3-527-30673-2.
- Thao; Yamakawa (2008). "Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator?". Soil Science and Plant Nutrition. 55 (2): 228–234. doi:10.1111/j.1747-0765.2009.00365.x. S2CID 95723306.
- "Phosphites and Phosphates: When Distributors and Growers alike could get confused!" by Jean-Pierre Leymonie. Courtesy of New Ag International, September 2007 edition.