Phosphorus trifluorodichloride
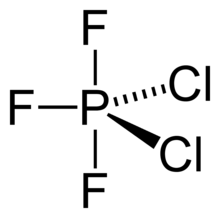
Phosphorus trifluorodichloride is a chemical compound with the chemical formula PF3Cl2. The covalent molecule trigonal bipyramidal molecular geometry. The central phosphorus atom has sp3d hybridization, and the molecule has an asymmetric charge distribution. It appears as a colorless gas with a disagreeable odor which turns to a liquid at -8 °C.
 | |
| Identifiers | |
|---|---|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| Cl2F3P | |
| Molar mass | 158.87 g·mol−1 |
| Appearance | Colorless gas |
| Related compounds | |
Other cations |
antimony trifluorodichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phosphorus trifluorodichloride is formed by mixing phosphorus trifluoride with chlorine PF3 + Cl2 → PF3Cl2[1]
The P-F bond length is 1.546 Å for equatorial position and 1.593 for the axial position and the P-Cl bond length is 2.004 Å. The chlorine atoms are in equatorial positions in the molecule.[1]
References
- French, Richard J.; Hedberg, Kenneth; Shreeve, Jeanne M.; Gupta, Krishna D. (August 1985). "Dichlorotrifluorophosphorane (PCl2F3): molecular structure by gas-phase electron diffraction and quadratic force field". Inorganic Chemistry. 24 (18): 2774–2777. doi:10.1021/ic00212a014.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.