Picrocrocin
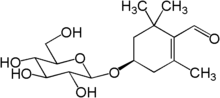
Picrocrocin is a monoterpene glycoside precursor of safranal. It is found in the spice saffron, which comes from the crocus flower.[1] Picrocrocin has a bitter taste, and is the chemical most responsible for the taste of saffron.
 | |
| Names | |
|---|---|
| IUPAC name
(4R)-4-(β-D-Glucopyranosyloxy)-2,6,6-trimethylcyclohex-1-ene-1-carbaldehyde | |
| Systematic IUPAC name
(4R)-2,6,6-Trimethyl-4-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}cyclohex-1-ene-1-carbaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H26O7 | |
| Molar mass | 330.37 g/mol |
| Density | 1.31 g/mL |
| Melting point | 154 to 156 °C (309 to 313 °F; 427 to 429 K) |
| Boiling point | 520.4 °C (968.7 °F; 793.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
During the drying process, picrocrocin liberates the aglycone (HTCC, C10H16O2) due to the action of the enzyme glucosidase. The aglycone is then transformed to safranal by dehydration. Picrocrocin is a degradation product of the carotenoid zeaxanthin.
References
- Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI (2007). "HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources". Food Chemistry. 100 (3): 1126–1131. doi:10.1016/j.foodchem.2005.11.020.
- Pfander, H.; Schurtenberger, H. (1982). "Biosynthesis of C20-carotenoids in Crocus sativus". Phytochemistry. 21 (5): 1039–1042. Bibcode:1982PChem..21.1039P. doi:10.1016/S0031-9422(00)82412-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.