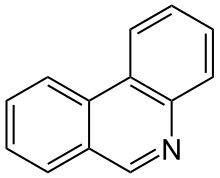
Phenanthridine
Phenanthridine is a nitrogen heterocyclic compound that is the basis of DNA-binding fluorescent dyes through intercalation. Examples of such dyes are ethidium bromide and propidium iodide. It is an isomer of acridine.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Phenanthridine[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.396 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H9N | |
| Molar mass | 179.217 g/mol |
| Melting point | 107.4 °C (225.3 °F; 380.5 K) |
| Boiling point | 348.9 °C (660.0 °F; 622.0 K) |
| slightly soluble[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
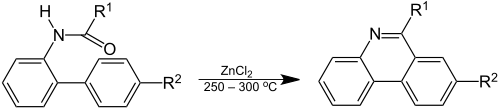
Phenanthridine was discovered by Amé Pictet and H. J. Ankersmit in 1891 by pyrolysis of the condensation product of benzaldehyde and aniline.[3] In the Pictet–Hubert reaction (1899) the compound is formed in a reaction of the 2-aminobiphenyl – formaldehyde adduct (an N-acyl-o-xenylamine) with zinc chloride at elevated temperatures.[4]
The reaction conditions for the Pictet–Hubert reaction were improved by Morgan and Walls in 1931, replacing the metal by phosphorus oxychloride and using nitrobenzene as a reaction solvent.[5] For this reason, the reaction is also called the Morgan–Walls reaction.[6]
The reaction is similar to the Bischler–Napieralski reaction and the Pictet–Spengler reaction.
References
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 212. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–460, ISBN 0-8493-0594-2
- Mittheilung Ueber das Phenanthridin Amé Pictet, H. J. Ankersmit Chemisches Laboratorium der Universität Genf Justus Liebigs Annalen der Chemie Volume 266 Issue 1–2, pp. 138–153 doi:10.1002/jlac.18912660107
- Mittheilungen Ueber eine neue Synthese der Phenanthridinbasen Amé Pictet, A. Hubert Berichte der deutschen chemischen Gesellschaft Volume 29 Issue 2, pp. 1182–1189, 1896 doi:10.1002/cber.18960290206
- CCCXXXV.—Researches in the phenanthridine series. Part I. A new synthesis of phenanthridine homologues and derivatives Gilbert T. Morgan, Leslie Percy Walls, J. Chem. Soc., 1931, 2447–2456 doi:10.1039/JR9310002447
- Jie Jack Li (ed.), 2004, Name Reactions in Heterocyclic Chemistry, Wiley.