Lithol Rubine BK
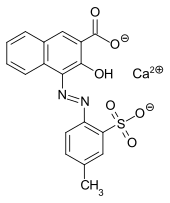
Lithol Rubine BK is a reddish synthetic azo dye. It has the appearance of a red powder and is magenta when printed. It is slightly soluble in hot water, insoluble in cold water, and insoluble in ethanol. When dissolved in dimethylformamide, its absorption maximum lies at about 442 nm. It is usually supplied as a calcium salt.[1] It is prepared by azo coupling with 3-hydroxy-2-naphthoic acid. It is used to dye plastics, paints, printing inks, and for textile printing. It is normally used as a standard magenta in the three and four color printing processes.
 | |
| Names | |
|---|---|
| IUPAC name
Calcium (4Z)-4-[(4-methyl-2-sulfonatophenyl)hydrazono]-3-oxo-2-naphthalenecarboxylate | |
| Other names
Pigment Rubine, Carmine 6B, Brilliant Carmine 6B, Permanent Rubin L6B, Litholrubine, Latolrubine, C.I. Pigment Red 57, C.I. Pigment Red 57:1, D&C Red No. 7, or C.I. 15850:1; E180 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.023.736 |
| E number | E180 (colours) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H12CaN2O6S | |
| Molar mass | 424.44 g/mol |
| Appearance | Red powder |
| slightly soluble in hot water, insoluble in cold water and ethanol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
When used as a food dye, it has E number E180. It is used to color cheese rind, and it is a component in some lip balms.
References
- K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.