PEDF
Pigment epithelium-derived factor (PEDF) also known as serpin F1 (SERPINF1), is a multifunctional secreted protein that has anti-angiogenic, anti-tumorigenic, and neurotrophic functions. Found in vertebrates, this 50 kDa protein is being researched as a therapeutic candidate for treatment of such conditions as choroidal neovascularization, heart disease, and cancer.[5] In humans, pigment epithelium-derived factor is encoded by the SERPINF1 gene.[6][7]
Discovery
Pigment epithelium-derived factor (PEDF) was originally discovered by Joyce Tombran-Tink and Lincoln Johnson in the late 1980s.[8][9] This group was studying human retinal cell development by identifying secreted factors produced by the retinal pigmented epithelium (RPE), a layer of cells that supports the retina. Upon noticing RPE produced a factor that promoted the differentiation of primitive retinal cells into cells of a neuronal phenotype, they set out to determine the identity of the factor. They isolated proteins unique to RPE cells and tested the individual proteins for neurotrophic function, meaning promoting a neuronal phenotype. A neurotrophic protein around 50 kilodaltons (kDa) was identified and temporarily named RPE-54 before being officially termed pigment epithelium-derived factor.
Soon thereafter, the same laboratory sequenced the PEDF protein and compared it to a human fetal eye library.[7] They found that PEDF was a previously uncharacterized protein and a member of the serpin (serine protease inhibitor) family.
Gene
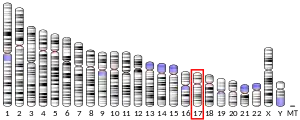
The gene encoding human PEDF was localized to the 17th chromosome at position 17p13.1.[10] The human PEDF gene is around 15.6kb, and the mRNA transcript is around 1.5kb.[11] Immediately upstream of the PEDF gene lies a 200bp promoter region with putative binding sites for the transcription factors HNF4, CHOP, and USF. The PEDF gene consists of 8 exons and 7 introns.
The PEDF gene is present in vertebrates from human to fish, but not present in sea squirts, worms, or fruit flies.[11] Sea squirts express several serpin genes, suggesting that the PEDF gene may have arisen from another serpin family member after the evolution of vertebral animals. The gene most homologous to PEDF is its adjacent neighbor on chromosome 17, SerpinF2.
Protein
The PEDF protein is a secreted protein of roughly 50kDa size and 418 amino acids in length.[5] The N-terminus contains a leader sequence responsible for protein secretion out of the cell at residues 1-19. A 34-mer fragment of PEDF (residues 24-57) was shown to have antiangiogenic properties, and a 44-mer (residues 58-101) was shown to have neurotrophic properties.[12] A BLAST search reveals a putative receptor binding site exists between residues 75-124. A nuclear localization sequence (NLS) exists about 150 amino acids into the protein. The additional molecular weight is partly due to a single glycosylation site at residue 285.[13] Near the C-terminus at residues 365-390 lies the reactive center loop (RCL) which is normally involved in serine protease inhibitor activity; however, in PEDF this region does not retain the inhibitory function.[5][14]
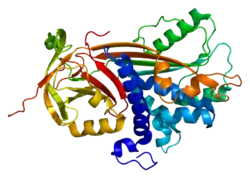
In 2001, the crystal structure of PEDF was successfully generated.[15] The PEDF structure includes 3 beta sheets and 10 alpha helices. This discovery demonstrated that PEDF has an asymmetrical charge distribution across the whole protein. One side of the protein is heavily basic and the other side is heavily acidic, leading to a polar 3-D structure. They proposed that the basic side of the protein contains a heparin binding site.
Signaling
PEDF expression is upregulated by plasminogen kringle domains 1-4 (also known as angiostatin) and the kringle 5 (K5) domain.[16][17] Hypoxia, or low oxygen conditions, leads to the downregulation of PEDF.[17] This effect is due to hypoxic conditions causing matrix metalloproteinases (MMPs) to proteolytically degrade PEDF.[18] In addition, amyloid beta has been shown to decrease PEDF mRNA levels.[19]
Secreted PEDF binds a receptor on the cell surface termed PEDF-R.[20] PEDF-R has phospholipase A2 activity which liberates fatty acids from glycerolipids. PEDF enhances gamma-secretase activity, leading to the cleavage of the VEGF receptor 1 (VEGFR-1) transmembrane domain.[21] This action interferes with VEGF signaling thereby inhibiting angiogenesis. Laminin receptor is also a target for PEDF, and the interaction occurs between residues 24-57 of PEDF, a region known to regulate antiangiogenic function.[22]
PEDF induces PPAR-gamma expression which in turn induces p53, a tumor suppressor gene involved in cell cycle regulation and apoptosis.[23] Thrombospondin, an antiangiogenic protein, is upregulated by PEDF.[24] PEDF stimulates several other well known signaling cascades such as the Ras pathway, the NF-κB pathway, and extrinsic apoptosis cascades.[25]
Function
PEDF has a variety of functions including antiangiogenic, antitumorigenic, and neurotrophic properties.[26] Endothelial cell migration is inhibited by PEDF.[27] PEDF suppresses retinal neovascularization and endothelial cell proliferation.[28][29] The antiangiogenic residues 24-57 were shown to be sufficient at inhibiting angiogenesis.[30] PEDF is also responsible for apoptosis of endothelial cells either through the p38 MAPK pathway[31] or through the FAS/FASL pathway[32] Antiangiogenic function is also conferred by PEDF through inhibition of both VEGFR-1[21] and VEGFR-2.[33]
The antitumorigenic effects of PEDF are not only due to inhibition of supporting vasculature, but also due to effects on the cancer cells themselves. PEDF was shown to inhibit cancer cell proliferation and increase apoptosis via the FAS/FASL pathway.[34] VEGF expression by cancer cells is inhibited by PEDF.[35]
PEDF also displays neurotrophic functions. Retinoblastoma cells differentiate into neurons due to the presence of PEDF.[9] Expression of PEDF in the human retina is found at 7.4 weeks of gestation, suggesting it may play a role in retinal neuron differentiation.[36]
Clinical significance
PEDF, a protein with many functions, has been suggested to play a clinical role in dry eye, choroidal neovascularization, cardiovascular disease, diabetes, diabetic macular edema, osteogenesis imperfecta and cancer.[37][26][28][30][38][39] As an antiangiogenic protein, PEDF may help suppress unwanted neovascularization of the eye. Molecules that shift the balance towards PEDF and away from VEGF may prove useful tools in both choroidal neovascularization and preventing cancer metastasis formation.[16][40][41]
References
- ENSG00000282307 GRCh38: Ensembl release 89: ENSG00000132386, ENSG00000282307 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000000753 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Filleur S, Nelius T, de Riese W, Kennedy RC (Apr 2009). "Characterization of PEDF: a multi-functional serpin family protein". Journal of Cellular Biochemistry. 106 (5): 769–75. doi:10.1002/jcb.22072. PMID 19180572. S2CID 30871963.
- "Entrez Gene: SERPINF1 serpin peptidase inhibitor, clade F (alpha-2 antiplasmin, pigment epithelium derived factor), member 1".
- Steele FR, Chader GJ, Johnson LV, Tombran-Tink J (Feb 1993). "Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family". Proceedings of the National Academy of Sciences of the United States of America. 90 (4): 1526–30. doi:10.1073/pnas.90.4.1526. PMC 45907. PMID 8434014.
- Tombran-Tink J, Johnson LV (Aug 1989). "Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells". Investigative Ophthalmology & Visual Science. 30 (8): 1700–7. PMID 2668219.
- Tombran-Tink J, Chader GG, Johnson LV (Sep 1991). "PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity". Experimental Eye Research. 53 (3): 411–4. doi:10.1016/0014-4835(91)90248-D. PMID 1936177.
- Tombran-Tink J, Pawar H, Swaroop A, Rodriguez I, Chader GJ (Jan 1994). "Localization of the gene for pigment epithelium-derived factor (PEDF) to chromosome 17p13.1 and expression in cultured human retinoblastoma cells". Genomics. 19 (2): 266–72. doi:10.1006/geno.1994.1057. hdl:2027.42/31831. PMID 8188257.
- Xu X, Zhang SS, Barnstable CJ, Tombran-Tink J (2006). "Molecular phylogeny of the antiangiogenic and neurotrophic serpin, pigment epithelium derived factor in vertebrates". BMC Genomics. 7: 248. doi:10.1186/1471-2164-7-248. PMC 1609119. PMID 17020603.
- Filleur S, Volz K, Nelius T, Mirochnik Y, Huang H, Zaichuk TA, Aymerich MS, Becerra SP, Yap R, Veliceasa D, Shroff EH, Volpert OV (Jun 2005). "Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer". Cancer Research. 65 (12): 5144–52. doi:10.1158/0008-5472.CAN-04-3744. PMID 15958558.
- Stratikos E, Alberdi E, Gettins PG, Becerra SP (Dec 1996). "Recombinant human pigment epithelium-derived factor (PEDF): characterization of PEDF overexpressed and secreted by eukaryotic cells". Protein Science. 5 (12): 2575–82. doi:10.1002/pro.5560051220. PMC 2143303. PMID 8976566.
- Becerra SP, Sagasti A, Spinella P, Notario V (Oct 1995). "Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop". The Journal of Biological Chemistry. 270 (43): 25992–9. doi:10.1074/jbc.270.43.25992. PMID 7592790.
- Simonovic M, Gettins PG, Volz K (Sep 2001). "Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor". Proceedings of the National Academy of Sciences of the United States of America. 98 (20): 11131–5. doi:10.1073/pnas.211268598. PMC 58695. PMID 11562499.
- Yang H, Xu Z, Iuvone PM, Grossniklaus HE (May 2006). "Angiostatin decreases cell migration and vascular endothelium growth factor (VEGF) to pigment epithelium derived factor (PEDF) RNA ratio in vitro and in a murine ocular melanoma model". Molecular Vision. 12: 511–7. PMID 16735992.
- Gao G, Li Y, Gee S, Dudley A, Fant J, Crosson C, Ma JX (Mar 2002). "Down-regulation of vascular endothelial growth factor and up-regulation of pigment epithelium-derived factor: a possible mechanism for the anti-angiogenic activity of plasminogen kringle 5". The Journal of Biological Chemistry. 277 (11): 9492–7. doi:10.1074/jbc.M108004200. PMID 11782462.
- Notari L, Miller A, Martínez A, Amaral J, Ju M, Robinson G, Smith LE, Becerra SP (Aug 2005). "Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia". Investigative Ophthalmology & Visual Science. 46 (8): 2736–47. doi:10.1167/iovs.04-1489. PMID 16043845.
- Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I (Oct 2005). "The potential role of amyloid beta in the pathogenesis of age-related macular degeneration". The Journal of Clinical Investigation. 115 (10): 2793–800. doi:10.1172/JCI24635. PMC 1201663. PMID 16167083.
- Notari L, Baladron V, Aroca-Aguilar JD, Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML, Sanchez-Sanchez F, Escribano J, Laborda J, Becerra SP (Dec 2006). "Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor". The Journal of Biological Chemistry. 281 (49): 38022–37. doi:10.1074/jbc.M600353200. PMID 17032652.
- Cai J, Jiang WG, Grant MB, Boulton M (Feb 2006). "Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1". The Journal of Biological Chemistry. 281 (6): 3604–13. doi:10.1074/jbc.M507401200. PMID 16339148.
- Bernard A, Gao-Li J, Franco CA, Bouceba T, Huet A, Li Z (Apr 2009). "Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor". The Journal of Biological Chemistry. 284 (16): 10480–90. doi:10.1074/jbc.M809259200. PMC 2667735. PMID 19224861.
- Ho TC, Chen SL, Yang YC, Liao CL, Cheng HC, Tsao YP (Nov 2007). "PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells". Cardiovascular Research. 76 (2): 213–23. doi:10.1016/j.cardiores.2007.06.032. PMID 17651710.
- Guan M, Pang CP, Yam HF, Cheung KF, Liu WW, Lu Y (May 2004). "Inhibition of glioma invasion by overexpression of pigment epithelium-derived factor". Cancer Gene Therapy. 11 (5): 325–32. doi:10.1038/sj.cgt.7700675. PMID 15044958.
- Tombran-Tink J, Barnstable CJ (Aug 2003). "PEDF: a multifaceted neurotrophic factor". Nature Reviews. Neuroscience. 4 (8): 628–36. doi:10.1038/nrn1176. PMID 12894238. S2CID 3113843.
- Rychli K, Huber K, Wojta J (Nov 2009). "Pigment epithelium-derived factor (PEDF) as a therapeutic target in cardiovascular disease". Expert Opinion on Therapeutic Targets. 13 (11): 1295–302. doi:10.1517/14728220903241641. PMID 19694500. S2CID 12373314.
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP (Jul 1999). "Pigment epithelium-derived factor: a potent inhibitor of angiogenesis". Science. 285 (5425): 245–8. doi:10.1126/science.285.5425.245. PMID 10398599.
- Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA (Aug 2001). "Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization". Journal of Cellular Physiology. 188 (2): 253–63. doi:10.1002/jcp.1114. PMID 11424092. S2CID 22379964.
- Duh EJ, Yang HS, Suzuma I, Miyagi M, Youngman E, Mori K, Katai M, Yan L, Suzuma K, West K, Davarya S, Tong P, Gehlbach P, Pearlman J, Crabb JW, Aiello LP, Campochiaro PA, Zack DJ (Mar 2002). "Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth". Investigative Ophthalmology & Visual Science. 43 (3): 821–9. PMID 11867604.
- Amaral J, Becerra SP (Mar 2010). "Effects of human recombinant PEDF protein and PEDF-derived peptide 34-mer on choroidal neovascularization". Investigative Ophthalmology & Visual Science. 51 (3): 1318–26. doi:10.1167/iovs.09-4455. PMC 2836227. PMID 19850839.
- Chen L, Zhang SS, Barnstable CJ, Tombran-Tink J (Oct 2006). "PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases". Biochemical and Biophysical Research Communications. 348 (4): 1288–95. doi:10.1016/j.bbrc.2006.07.188. PMID 16919597.
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP (Apr 2002). "Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor". Nature Medicine. 8 (4): 349–57. doi:10.1038/nm0402-349. PMID 11927940. S2CID 9867297.
- Zhang SX, Wang JJ, Gao G, Parke K, Ma JX (Aug 2006). "Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy". Journal of Molecular Endocrinology. 37 (1): 1–12. doi:10.1677/jme.1.02008. PMID 16901919.
- Garcia M, Fernandez-Garcia NI, Rivas V, Carretero M, Escamez MJ, Gonzalez-Martin A, Medrano EE, Volpert O, Jorcano JL, Jimenez B, Larcher F, Del Rio M (Aug 2004). "Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor". Cancer Research. 64 (16): 5632–42. doi:10.1158/0008-5472.CAN-04-0230. PMID 15313901.
- Takenaka K, Yamagishi S, Jinnouchi Y, Nakamura K, Matsui T, Imaizumi T (Nov 2005). "Pigment epithelium-derived factor (PEDF)-induced apoptosis and inhibition of vascular endothelial growth factor (VEGF) expression in MG63 human osteosarcoma cells". Life Sciences. 77 (25): 3231–41. doi:10.1016/j.lfs.2005.05.048. PMID 15985268.
- Karakousis PC, John SK, Behling KC, Surace EM, Smith JE, Hendrickson A, Tang WX, Bennett J, Milam AH (Jun 2001). "Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues". Molecular Vision. 7: 154–63. PMID 11438800.
- Singh, Rohan Bir; Blanco, Tomas; Mittal, Sharad K.; Taketani, Yukako; Chauhan, Sunil K.; Chen, Yihe; Dana, Reza (2020). "Pigment Epithelium-derived Factor secreted by corneal epithelial cells regulates dendritic cell maturation in dry eye disease". The Ocular Surface. 18 (3): 460–469. doi:10.1016/j.jtos.2020.05.002. ISSN 1937-5913. PMC 7322788. PMID 32387568.
- Funatsu H, Yamashita H, Nakamura S, Mimura T, Eguchi S, Noma H, Hori S (Feb 2006). "Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema". Ophthalmology. 113 (2): 294–301. doi:10.1016/j.ophtha.2005.10.030. PMID 16406543.
- Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, Bergmann C, Rohrbach M, Koerber F, Zimmermann K, de Vries P, Wirth B, Schoenau E, Wollnik B, Veltman JA, Hoischen A, Netzer C (Mar 2011). "Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta". American Journal of Human Genetics. 88 (3): 362–71. doi:10.1016/j.ajhg.2011.01.015. PMC 3059418. PMID 21353196.
- Tong JP, Yao YF (Mar 2006). "Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions". Clinical Biochemistry. 39 (3): 267–76. doi:10.1016/j.clinbiochem.2005.11.013. PMID 16409998.
- Yang H, Akor C, Dithmar S, Grossniklaus HE (Dec 2004). "Low dose adjuvant angiostatin decreases hepatic micrometastasis in murine ocular melanoma model". Molecular Vision. 10: 987–95. PMID 15623988.