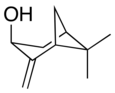
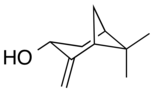
Pinocarveol
Pinocarveol is an organic compound with the formula C10H16O. It is a bicyclic monoterpenoid, which is a combination of two isoprene units with one hydroxyl group as a substituent.[1] It exists as either trans- or cis-pinocarveol, referring to stereochemical orientation of the oxygen as compared to the methylene bridge. It is a naturally occurring molecule in numerous plant species including Eucalyptus globulus and Picea abies.[2] Pinocarveol is found in a variety of essential oils.[3][4]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
6,6-dimethyl-2-methylidenebicyclo[3.1.1]heptan-3-ol | |||
| Other names
6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptan-3-ol 10-pinen-3-ol Isopinocarveol (1S,3R,5S)-2(10)-Pinen-3-ol | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.025.187 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H16O | |||
| Molar mass | 152.237 g·mol−1 | ||
| Appearance | Light yellow viscous liquid | ||
| Odor | woody | ||
| Density | 0.9730 g/cm3 | ||
| Boiling point | 217 °C (423 °F; 490 K) | ||
| insoluble in water | |||
| Solubility | soluble in ethanol, soluble in oils | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 90.1°C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Synthesis
Pinocarveol is formed by heating a mixture of turpentine, selenium dioxide, and hydrogen peroxide. The selenium dioxide acts as a catalyst while the hydrogen peroxide oxidizes the pinene found in turpentine. The other products in the turpentine are left unreacted.[5]
References
- Clarke, S (2008), "Families of compounds that occur in essential oils", Essential Chemistry for Aromatherapy, Elsevier, pp. 41–77, doi:10.1016/b978-0-443-10403-9.00003-0, ISBN 978-0-443-10403-9
- "LOTUS: Natural Products Online". lotus.naturalproducts.net. Retrieved 2022-02-22.
- Dob, T.; Dahmane, D.; Chelghoum, C. (2006-01-01). "Essential Oil Composition of Juniperus Oxycedrus. Growing in Algeria". Pharmaceutical Biology. 44 (1): 1–6. doi:10.1080/13880200500530922. ISSN 1388-0209. S2CID 84516218.
- Bansal, Anita; Boehme, Amelia K.; Eiter, Lauren C.; Schmidt, Jennifer M.; Setzer, William N.; Vincent, Michael A. (2006). "Chemical Composition and Bioactivity of the Leaf Oil of Calyptranthes pallens (Poir.) Griseb. from Abaco Island, Bahamas". Natural Product Communications. 1 (4): 1934578X0600100. doi:10.1177/1934578X0600100407. ISSN 1934-578X. S2CID 132063891.
- Coxon, James M.; Dansted, Erik; Hartshorn, Michael P. (1970). "trans-Pinocarveol from New Zealand turpentine". Journal of Chemical & Engineering Data. 15 (2): 336. doi:10.1021/je60045a013. ISSN 0021-9568.
- "Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) N 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC – Text with EEA relevance". 2 October 2012.