Poloxamer 407
Poloxamer 407 is a hydrophilic non-ionic surfactant of the more general class of copolymers known as poloxamers. Poloxamer 407 is a triblock copolymer consisting of a central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol (PEG). The approximate lengths of the two PEG blocks is 101 repeat units, while the approximate length of the propylene glycol block is 56 repeat units.[1] This particular compound is also known by the BASF trade name Pluronic F-127 or by the Croda trade name Synperonic PE/F 127. BASF also offers a pharmaceutical grade, under trade name Kolliphor P 407.[2]
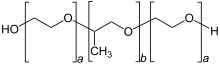 Skeleton formula of poloxameres, where poloxamer 407 has block lengths of a = 101 and b = 56 | |
| Names | |
|---|---|
| IUPAC name
Oxirane, methyl-, polymer with oxirane | |
Other names
| |
| Identifiers | |
| DrugBank | |
PubChem CID |
|
| UNII | |
| Properties | |
| C 572H 1146O 259 | |
| Molar mass | 12,600 g/mol |
| Appearance | white powder |
| Melting point | 53–57 °C (127–135 °F; 326–330 K) |
| very soluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | Kolliphor P 407 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
Most of the common uses of poloxamer 407 are related to its surfactant properties. For example, it is widely used in cosmetics for dissolving oily ingredients in water. It can also be found in multi-purpose contact lens cleaning solutions, where its purpose there is to help remove lipid films from the lens. It can also be found in some mouthwashes. There is research ongoing for using poloxamer 407 for aligning severed blood vessels before gluing them surgically.[3] Poloxamer 407 can also be used for its thermogelling properties in aqueous media.
Poloxamer 407 is approved by the FDA for use as an excipient in a range of pharmaceutical dosage forms, and is listed in the Inactive Ingredient Database (IID).[4]
Poloxamer 407 is used in bioprinting applications due to its unique phase-change properties.[5] In a 30% solution by weight, poloxamer 407 forms a gel solid at room temperature but liquifies when chilled to 4 °C (39 °F). This allows poloxamer 407 to serve as a removable support material, particularly for creating hollow channels or cavities inside hydrogels.[6][7] In this role, it is often referred to as a "sacrificial ink" or a "fugitive ink".
Reports of adverse effects
It was reported in The Australian newspaper 18 November 2006 that this common ingredient in toothpaste and mouthwash can cause high cholesterol in mice.[8] A team from the Centre for Ageing and the ANZAC Research Institute in Sydney used it as a tool to demonstrate that cells in the liver behave like a sieve. They gave a high dose (1 gram per kilogram of body weight) of poloxamer 407 to mice, which blocked 80% of the pores in liver cells that absorb lipoproteins, leading to a 10-fold increase in plasma lipid levels.[9] However, the dose used is far higher than a person would be exposed to in toothpaste or mouthwash.
Potential degradation by sonication
Wang et al.[10] reported that aqueous solutions of poloxamer 188 and poloxamer 407 sonicated in the presence or absence of multi-walled carbon nanotubes (MWNTs) can become highly toxic to cultured cells. The toxicity correlated with the sonolytic degradation of the polymers.
References
- Tania Betancourt; The University of Texas at Austin. Biomedical Engineering (2007). Targetable biodegradable nanoparticles for delivery of chemotherapeutic and imaging agents to ovarian cancer. ProQuest. pp. 130–. ISBN 978-0-549-34761-3. Retrieved 16 August 2011.
- "Poloxamers for Pharmaceutical Applications". BASF Pharma. Retrieved 2022-06-11.
- Stanford University Medical Center (28 August 2011). "Sutureless method for joining blood vessels invented". ScienceDaily.
- "Inactive Ingredient Search for Approved Drug Products". www.accessdata.fda.gov. Retrieved 2022-06-11.
- Gopinathan, Janarthanan (2018). "Recent trends in bioinks for 3D printing". Biomaterials Research. 22 (1): 11. doi:10.1186/s40824-018-0122-1. PMC 5889544. PMID 29636985.
- Homan, Kimberly A.; Lewis, Jennifer A (2016). "Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips". Scientific Reports. 6: 34845. doi:10.1038/srep34845. PMC 5057112. PMID 27725720.
- Kang, Hyun-Wook; Atala, Anthony (2016). "A 3D bioprinting system to produce human-scale tissue constructs with structural integrity". Nature Biotechnology. 34 (3): 312–9. doi:10.1038/nbt.3413. PMID 26878319.
- O'Neill, Craig (18 November 2006). "Dental hygiene gives you a brush with cholesterol". The Australian.
- Cogger, VC; Hilmer, SN; Sullivan, D; Muller, M; Fraser, R; Le Couteur, DG (December 2006). "Hyperlipidemia and surfactants: the liver sieve is a link". Atherosclerosis. 189 (2): 273–81. doi:10.1016/j.atherosclerosis.2005.12.025. PMID 16458315.
- Wang, Ruhung; Hughes, Tyler; Beck, Simon; Vakil, Samee; Li, Synyoung; Pantano, Paul; Draper, Rockford K. (2013). "Generation of toxic degradation products by sonication of Pluronic® dispersants: implications for nanotoxicity testing". Nanotoxicology. 7 (7): 1272–1281. doi:10.3109/17435390.2012.736547. ISSN 1743-5390. PMC 3657567. PMID 23030523.
